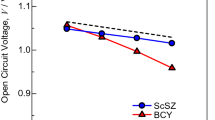
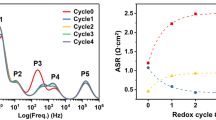
Abstract
High-temperature co-electrolysis is studied on electrolyte-supported NiO-YSZ/NiO-SDC/ScSZ/LSCF-GDC/LSCF (NiO: Nickel Oxide, YSZ: Yttria-stabilized zirconia, SDC: Samarium-doped ceria, ScSZ: Scandia-stabilized zirconia, LSCF: Lanthanum Strontium Cobalt Ferrite, GDC: Gadolinium-doped ceria) button cell. Electrochemical impedance spectroscopy (EIS) was recorded under open-circuit voltage (OCV) and co-electrolysis mode over various operating conditions, including temperature, water vapor content, and applied voltage. Interfacial polarization resistance (Rp) is obtained from peak arcs located in the three regions: gas conversion resistance (Region I (0.01 to 0.1 Hz)), gas diffusion resistance (Region II (0.1 to 100 Hz)) and air electrode charge transfer resistance (Region III (100 to 10,000 Hz)). As the temperature increased from 700 to 850 oC, Rp decreased from 18.15 to 3.32 Ω.cm2 at 1.3 V for 10%CO2/3%H2O. From the Distribution of relaxation times (DRT) studies, one additional peak, P5 (fuel gas conversion or gas-phase diffusion in the pores of the air electrode), is observed, and Region III (100 to 10,000 Hz) consists of two additional peaks: P1 (ionic transport coupled with gas diffusion close to triple phase boundaries (TPBs)) and P2 (fuel electrode charge transfer reaction), which were not clearly distinguished from EIS. Region II dominates in the overall polarization resistance. At 800 oC, for 10%CO2/3%H2O, the Rp decreased from 6.78 to 4.82 Ω.cm2, with an increase in the applied voltage from 1.3 to 1.5 V. At 800oC/1.5 V, the Rp values are 4.41, 8.09, and 6.77 Ω.cm2 for H2O, CO2, and co-electrolysis. At 800 ºC/1.5 V, with an increase in the water vapor content from 3%H2O to 15%H2O, there is not much change in the Rp value; therefore, 10%H2O is sufficient. H2 consumption is between 23 and 36%, depending on the temperature at OCV. At 800 °C for (10%H2/10%CO2/10%H2O), co-electrolysis occurs at applied voltage, along with Reverse water gas shift (RWGS) reaction.
Graphical Abstract









Similar content being viewed by others
Data availability
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
References
Graves C, Ebbesen SD, Mogensen M, Lackner KS (2011) Sustainable hydrocarbon fuels by recycling CO2 and H2O with renewable or nuclear energy. Renew Sustain Energy Rev 15:1–23. https://doi.org/10.1016/j.rser.2010.07.014
Fu Q, Mabilat C, Zahid M et al (2010) Syngas production via high-temperature steam/CO2 co-electrolysis: an economic assessment. Energy Environ Sci 3:1382–1397. https://doi.org/10.1039/c0ee00092b
Zheng Y, Wang J, Yu B et al (2017) A review of high temperature co-electrolysis of H2O and CO2 to produce sustainable fuels using solid oxide electrolysis cells (SOECs): Advanced materials and technology. Chem Soc Rev 46:1427–1463. https://doi.org/10.1039/c6cs00403b
Zhang X, Song Y, Wang G, Bao X (2017) Co-electrolysis of CO2 and H2O in high-temperature solid oxide electrolysis cells: recent advance in cathodes. J Energy Chem 26:839–853. https://doi.org/10.1016/j.jechem.2017.07.003
Li W, Wang H, Shi Y, Cai N (2013) Performance and methane production characteristics of H2O-CO2 co-electrolysis in solid oxide electrolysis cells. Int J Hydrogen Energy 38:11104–11109. https://doi.org/10.1016/j.ijhydene.2013.01.008
Neidhardt JP, Kee RJ, Bessler WG (2013) Electrode reoxidation in Solid-Oxide cells: detailed modeling of Nickel Oxide Film Growth. ECS Trans 57:2573–2582. https://doi.org/10.1149/05701.2573ecst
Torrell M, García-Rodríguez S, Morata A et al (2015) Co-electrolysis of steam and CO2 in full-ceramic symmetrical SOECs: a strategy for avoiding the use of hydrogen as a safe gas. Faraday Discuss 182:241–255. https://doi.org/10.1039/c5fd00018a
Henke M, Hillius S, Riedel M et al (2016) Gas recirculation at the Hydrogen Electrode of Solid Oxide Fuel Cell and Solid Oxide Electrolysis Cell systems. Fuel Cells 16:584–590. https://doi.org/10.1002/fuce.201500114
Cooper SJ, Bertei A, Finegan DP, Brandon NP (2017) Simulated impedance of diffusion in porous media. Electrochim Acta 251:681–689. https://doi.org/10.1016/j.electacta.2017.07.152
Zhang Y, Chen Y, Li M et al (2016) A high-precision approach to reconstruct distribution of relaxation times from electrochemical impedance spectroscopy. J Power Sources 308:1–6. https://doi.org/10.1016/j.jpowsour.2016.01.067
Boukamp BA (2004) Electrochemical impedance spectroscopy in solid state ionics: recent advances. Solid State Ionics 169:65–73. https://doi.org/10.1016/j.ssi.2003.07.002
Liu J, Ciucci F (2020) The gaussian process distribution of relaxation times: a machine learning tool for the analysis and prediction of electrochemical impedance spectroscopy data. Electrochim Acta 331. https://doi.org/10.1016/j.electacta.2019.135316
Zhang Y, Chen Y, Yan M, Chen F (2015) Reconstruction of relaxation time distribution from linear electrochemical impedance spectroscopy. J Power Sources 283:464–477. https://doi.org/10.1016/j.jpowsour.2015.02.107
Schichlein H, Müller AC, Voigts M et al (2002) Deconvolution of electrochemical impedance spectra for the identification of electrode reaction mechanisms in solid oxide fuel cells. J Appl Electrochem 32:875–882. https://doi.org/10.1023/A:1020599525160
Klotz D, Schönleber M, Schmidt JP, Ivers-Tiffée E (2011) New approach for the calculation of impedance spectra out of time domain data. Electrochim Acta 56:8763–8769. https://doi.org/10.1016/j.electacta.2011.07.096
Bertei A, Ruiz-Trejo E, Tariq F et al (2016) Validation of a physically-based solid oxide fuel cell anode model combining 3D tomography and impedance spectroscopy. Int J Hydrogen Energy 41:22381–22393. https://doi.org/10.1016/j.ijhydene.2016.09.100
Zhang Y, Chen Y, Chena F (2015) In-situ quantification of solid oxide fuel cell electrode microstructure by electrochemical impedance spectroscopy. J Power Sources 277:277–285. https://doi.org/10.1016/j.jpowsour.2014.11.123
Lyagaeva JG, Vdovin GK, Medvedev DA (2019) Distinguishing bulk and Grain Boundary Transport of a Proton-conducting Electrolyte by Combining Equivalent Circuit Scheme and Distribution of Relaxation Times Analyses. J Phys Chem C 123:21993–21997. https://doi.org/10.1021/acs.jpcc.9b05705
Riedel M, Heddrich MP, Ansar A et al (2020) Pressurized operation of solid oxide electrolysis stacks: an experimental comparison of the performance of 10-layer stacks with fuel electrode and electrolyte supported cell concepts. J Power Sources 475. https://doi.org/10.1016/j.jpowsour.2020.228682
Mahmood A, Bano S, Yu JH, Lee KH (2019) Performance evaluation of SOEC for CO2/H2O co-electrolysis: considering the effect of cathode thickness.J CO2 utilization 33:114 – 20. https://doi.org/10.1016/j.jcou.2019.05.014
Jeong HY, Yoon KJ, Lee JH et al (2018) Long-term stability for co-electrolysis of CO2/steam assisted by catalyst-infiltrated solid oxide cells. J Korean Ceram Soc 55:50–54. https://doi.org/10.4191/kcers.2018.55.1.09
Zhou J, Ma Z, Zhang L et al (2019) Study of CO2 and H2O direct co-electrolysis in an electrolyte-supported solid oxide electrolysis cell by aqueous tape casting technique. Int J Hydrogen Energy 44:28939–28946. https://doi.org/10.1016/j.ijhydene.2019.09.141
Xia J, Wang C, Wang X et al (2020) A perspective on DRT applications for the analysis of solid oxide cell electrodes. Electrochim Acta 349. https://doi.org/10.1016/j.electacta.2020.136328
Wan TH, Saccoccio M, Chen C, Ciucci F (2015) Influence of the discretization methods on the distribution of relaxation Times Deconvolution: implementing Radial basis functions with DRTtools. Electrochim Acta 184:483–499. https://doi.org/10.1016/j.electacta.2015.09.097
Li Y, Singh M, Zhuang Z et al (2021) Efficient reversible CO/CO2 conversion in solid oxide cells with a phase-transformed fuel electrode. Sci China Mater 64:1114–1126. https://doi.org/10.1007/s40843-020-1531-7
Li Y, Li Y, Zhang S et al (2022) Mutual Conversion of CO-CO2 on a Perovskite fuel electrode with endogenous Alloy nanoparticles for reversible solid oxide cells. ACS Appl Mater Interfaces 14:9138–9150. https://doi.org/10.1021/acsami.1c23548
Lin W, Su W, Li Y et al (2023) Enhancing Electrochemical CO2 reduction on Perovskite Oxide for Solid Oxide Electrolysis Cells through in situ A-Site deficiencies and Surface Carbonate Deposition Induced by Lithium Cation Doping and Exsolution. Small 19:1–11. https://doi.org/10.1002/smll.202303305
Lyu Z, Li H, Wang Y, Han M (2021) Performance degradation of solid oxide fuel cells analyzed by evolution of electrode processes under polarization. J Power Sources 485. https://doi.org/10.1016/j.jpowsour.2020.229237
Leonide A, Apel Y, Ivers-Tiffee E (2009) SOFC modeling and parameter identification by means of Impedance Spectroscopy. ECS Meet Abstr MA2009–01:1439–1439. https://doi.org/10.1149/ma2009-01/42/1439
Leonide A, Sonn V, Weber A, Ivers-Tiffée E (2008) Evaluation and modeling of the Cell Resistance in Anode-supported solid oxide fuel cells. J Electrochem Soc 155:B36. https://doi.org/10.1149/1.2801372
Yue X, Irvine JTS (2012) Impedance studies on LSCMGDC cathode for high temperature CO2 electrolysis. Electrochem Solid-State Lett 15:31–34. https://doi.org/10.1149/2.021203esl
Khameneh MK, Babaei A (2019) Co-electrolysis of CO2 and H2O on LaFe0. 6Co0.4O3 promoted La0.75Sr0.25Cr0.5Mn0.5O3/YSZ electrode in solid oxide electrolysis cell. Electrochim Acta 299:132–142. https://doi.org/10.1016/j.electacta.2018.12.136
Ma Z, Li Y, Zheng Y et al (2021) La0. 75Sr0. 25Cr0. 5Mn0. 5O3–δ as cathode for electrolysis and co-electrolysis of CO2 and H2O in solid oxide electrolysis cell. Ceram Int 47(16):23350–23361. https://doi.org/10.1016/j.ceramint.2021.05.049
Yang Y, Wang Y, Yang Z et al (2020) A highly active and durable electrode with in situ exsolved Co nanoparticles for solid oxide electrolysis cells. J Power Sources 478:1–8. https://doi.org/10.1016/j.jpowsour.2020.229082
Chen L, Xu J, Wang X, Xie K (2020) Sr2Fe1. 5+ xMo0. 5O6–δ cathode with exsolved Fe nanoparticles for enhanced CO2 electrolysis. Int J Hydrogen Energy 5:4–10. https://doi.org/10.1016/j.ijhydene.2020.02.140
Ebbesen SD, Knibbe R, Mogensen M (2012) Co-electrolysis of Steam and Carbon Dioxide in Solid Oxide cells. J Electrochem Soc 159:F482–F489. https://doi.org/10.1149/2.076208jes
Zhang W, Zheng Y, Yu B et al (2017) Electrochemical characterization and mechanism analysis of high temperature co-electrolysis of CO2 and H2O in a solid oxide electrolysis cell. Int J Hydrogen Energy 42:29911–29920. https://doi.org/10.1016/j.ijhydene.2017.06.225
Gewies S, Bessler WG, Sonn V, Ivers-Tiffee E (2007) Experimental and modeling study of the Impedance of Ni/YSZ Cermet Anodes. ECS Trans 7:1573–1582. https://doi.org/10.1149/1.2729264
Zhu H, Kromp A, Leonide A et al (2012) A model-based interpretation of the influence of Anode Surface Chemistry on Solid Oxide Fuel Cell Electrochemical Impedance Spectra. J Electrochem Soc 159:F255–F266. https://doi.org/10.1149/2.046207jes
Bienen F, Kopljar D, Löwe A, Geiger S et al (2020) Revealing mechanistic processes in gas-diffusion electrodes during CO2 reduction via impedance spectroscopy. ACS Sustain Chem Eng 8(36):13759–13768. https://doi.org/10.1021/acssuschemeng.0c04451
Dasari HP, Park SY, Kim J et al (2013) Electrochemical characterization of Ni-yttria stabilized Zirconia electrode for hydrogen production in solid oxide electrolysis cells. J Power Sources 240:721–728. https://doi.org/10.1016/j.jpowsour.2013.05.033
Kirtley JD, Halat DM, McIntyre MD et al (2012) High-temperature spectrochronopotentiometry: correlating electrochemical performance with in situ Raman spectroscopy in solid oxide fuel cells. Anal Chem 84:9745–9753. https://doi.org/10.1021/ac301504g
Shearing PR, Bradley RS, Gelb J et al (2012) Exploring microstructural changes associated with oxidation in Ni-YSZ SOFC electrodes using high resolution X-ray computed tomography. Solid State Ionics 216:69–72. https://doi.org/10.1016/j.ssi.2011.10.015
Nelson GJ, Harris WM, Izzo JR et al (2011) Three-dimensional mapping of nickel oxidation states using full field x-ray absorption near edge structure nanotomography. Appl Phys Lett 98. https://doi.org/10.1063/1.3574774
Yoon KJ, Coyle CA, Marina OA (2011) Doped yttrium chromite-ceria composite as a redox-stable and sulfur-tolerant anode for solid oxide fuel cells. Electrochem Commun 13:1400–1403. https://doi.org/10.1016/j.elecom.2011.08.025
Lo Faro M, Zignani SC, Trocino S et al (2019) New insights on the co-electrolysis of CO2 and H2O through a solid oxide electrolyser operating at intermediate temperatures. Electrochim Acta 296:458–464. https://doi.org/10.1016/j.electacta.2018.11.079
Yu S, Bin, Lee SH, Mehran MT et al (2018) Syngas production in high performing tubular solid oxide cells by using high-temperature H2O/CO2 co-electrolysis. Chem Eng J 335:41–51. https://doi.org/10.1016/j.cej.2017.10.110
Stoots C, O’Brien J, Hartvigsen J (2009) Results of recent high temperature coelectrolysis studies at the Idaho National Laboratory. Int J Hydrogen Energy 34:4208–4215. https://doi.org/10.1016/j.ijhydene.2008.08.029
Kim-Lohsoontorn P, Bae J (2011) Electrochemical performance of solid oxide electrolysis cell electrodes under high-temperature coelectrolysis of steam and carbon dioxide. J Power Sources 196:7161–7168. https://doi.org/10.1016/j.jpowsour.2010.09.018
Graves C, Ebbesen SD, Mogensen M (2011) Co-electrolysis of CO2 and H2O in solid oxide cells: performance and durability. Solid State Ionics 192:398–403. https://doi.org/10.1016/j.ssi.2010.06.014
Ebbesen SD, Graves C, Mogensen M (2009) Production of synthetic fuels by co-electrolysis of steam and carbon dioxide. Int J Green Energy 6:646–660. https://doi.org/10.1080/15435070903372577
Acknowledgements
This research work is funded by the SERB-IMPRINT-II (IMP/2018/001318) project titled “Development and demonstration of solid oxide electrolysis cell technology for co-electrolysis CO2 and H2O for the production of syngas”.
Author information
Authors and Affiliations
Contributions
RS: Conceptualization, Investigation, Validation, Resources, Writing, Review, and Editing original draft.
HPD: Conceptualization, Supervision, Validation, Investigation, Funding acquisition, Resources, Writing, Review, and Editing.
M.B.S: Funding acquisition, Writing, Review, and Editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shirasangi, R., Dasari, H. & Saidutta, M. Electrochemical characterization of electrolyte supported solid oxide electrolysis cell during CO2/H2O co-electrolysis. J Solid State Electrochem (2024). https://doi.org/10.1007/s10008-024-05853-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10008-024-05853-2