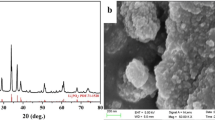
Abstract
1,1,2,2-Tetrafluoroethyl-2,2,3,3-tetrafluoropropyl ether (F-EPE) is investigated as a cosolvent for high voltage electrolytes of Li2CoPO4F. Compared with conventional carbonate-based electrolyte (1-M LiPF6 ethylene carbonate [EC]/dimethyl carbonate [DMC] [1:1, wt:wt]), 1 M LiPF6 F-EPE/DMC (1:2, wt:wt) exhibits significantly improved antioxidant ability in high voltage, thus greatly enhances the electrochemical performance of 5.0-V Li2CoPO4F/Li cells. Linear sweep voltammetry (LSV) and charging/discharging tests demonstrate that the F-EPE/DMC electrolyte possesses both a high oxidation voltage up to 6.2 V vs. Li+/Li on Pt electrode and superior oxidation stability on Li2CoPO4F cathode. Benefiting from its high antioxidant ability, the capacity retention of Li2CoPO4F cathode increases from 15% in EC/DMC electrolyte to 51% in F-EPE/DMC electrolyte after 100 cycles at 1 C between 3.0 and 5.4 V. Moreover, differential capacity (dQ/dV) analysis, electrochemical impedance spectroscopy, ex situ X-ray diffraction, and X-ray photoelectron spectroscopy are used to analyze the effects of F-EPE/DMC electrolyte on the improved electrochemical performance. It is illustrated that the high stability of F-EPE/DMC electrolyte effectively inhibits the oxidative decomposition of the electrolyte on Li2CoPO4F electrode above 5.0 V and suppresses the damage to the surface of Li2CoPO4F, thus alleviate the increase in electrode polarization and cell impedance.








Similar content being viewed by others
References
Fedotov SS, Kabanov AA, Kabanova NA, Blatov VA, Zhugayevych A, Abakumov AM, Khasanova NR, Antipov EV (2017) Crystal structure and Li-ion transport in Li2CoPO4F high-voltage cathode material for Li-ion batteries. J Phys Chem C 121(6):3194–3202. https://doi.org/10.1021/acs.jpcc.6b11027
Wang D, Xiao J, Xu W, Nie Z, Wang C, Graff G, Zhang J-G (2011) Preparation and electrochemical investigation of Li2CoPO4F cathode material for lithium-ion batteries. J Power Sources 196(4):2241–2245. https://doi.org/10.1016/j.jpowsour.2010.10.021
Okada S, Ueno M, Uebou Y, Yamaki J (2005) Fluoride phosphate Li2COPO4F as a high-voltage cathode in Li-ion batteries. J Power Sources 146(1-2):565–569. https://doi.org/10.1016/j.jpowsour.2005.03.149
Ortiz GF, Lopez MC, Li YX, McDonald MJ, Cabello M, Tirado JL, Yang Y (2016) Enhancing the energy density of safer Li-ion batteries by combining high-voltage lithium cobalt fluorophosphate cathodes and nanostructured titania anodes. Sci Rep 6(1):8. https://doi.org/10.1038/srep20656
Okumura T, Shikano M, Yamaguchi Y, Kobayashi H (2015) Structural changes in Li2CoPO4F during lithium-ion battery reactions. Chem Mater 27(8):2839–2847. https://doi.org/10.1021/cm504633p
Quang Duc T, Devaraju MK, Ganbe Y, Tomai T, Honma I (2014) Structural analysis and electrochemical performance of Li2CoPO4F cathode materials. Electrochim Acta 127:245–251. https://doi.org/10.1016/j.electacta.2014.02.026
Wu XB, Gong ZL, Tan S, Yang Y (2012) Sol-gel synthesis of Li2CoPO4F/C nanocomposite as a high power cathode material for lithium ion batteries. J Power Sources 220:122–129. https://doi.org/10.1016/j.jpowsour.2012.07.099
Khasanova NR, Gavrilov AN, Antipov EV, Bramnik KG, Hibst H (2011) Structural transformation of Li2CoPO4F upon Li-deintercalation. J Power Sources 196(1):355–360. https://doi.org/10.1016/j.jpowsour.2010.06.086
Ellis BL, Makahnouk WRM, Makimura Y, Toghill K, Nazar LF (2007) A multifunctional 3.5 V iron-based phosphate cathode for rechargeable batteries. Nat Mater 6(10):749–753. https://doi.org/10.1038/nmat2007
Recham N, Chotard JN, Dupont L, Djellab K, Armand M, Tarascon JM (2009) Ionothermal synthesis of sodium-based fluorophosphate cathode materials. J Electrochem Soc 156(12):A993–A999. https://doi.org/10.1149/1.3236480
Fedotov SS, Aksyonov DA, Samarin AS, Karakulina OM, Hadermann J, Stevenson KJ, Khasanova NR, Abakumov AM, Antipov EV (2019) Tuning the crystal structure of A(2)CoPO(4)F (A = Li, Na) fluoride-phosphates: a new layered polymorph of LiNaCoPO4F. Eur J Inorg Chem 2019(39-40):4365–4372. https://doi.org/10.1002/ejic.201900660
Xu K (2014) Electrolytes and interphases in Li-ion batteries and beyond. Chem Rev 114(23):11503–11618. https://doi.org/10.1021/cr500003w
Schoiber J, Berger RJF, Yada C, Miki H, Huesing N (2015) A two-step synthesis for Li2CoPO4F as high-voltage cathode material. J Electrochem Soc 162(14):A2679–A2683. https://doi.org/10.1149/2.0331514jes
Amaresh S, Karthikeyan K, Kim KJ, Kim MC, Chung KY, Cho BW, Lee YS (2013) Facile synthesis of ZrO2 coated Li2CoPO4F cathode materials for lithium secondary batteries with improved electrochemical properties. J Power Sources 244:395–402. https://doi.org/10.1016/j.jpowsour.2012.12.010
Amaresh S, Karthikeyan K, Kim KJ, Nahm KS, Lee YS (2014) Alumina coating on 5 V lithium cobalt fluorophosphate cathode material for lithium secondary batteries–synthesis and electrochemical properties. RSC Adv 4(44):23107–23115. https://doi.org/10.1039/c4ra02318h
Wu X, Wang S, Lin X, Zhong G, Gong Z, Yang Y (2014) Promoting long-term cycling performance of high-voltage Li2CoPO4F by the stabilization of electrode/electrolyte interface. J Mater Chem A 2(4):1006–1013. https://doi.org/10.1039/C3TA13801A
Chang C, Huang Z, Tian R, Jiang X, Li C, Feng J (2017) Targeted partial surface modification with nano-SiO2@Li2CoPO4F as high-voltage cathode material for LIBs. J Power Sources 364:351–358. https://doi.org/10.1016/j.jpowsour.2017.08.047
Yuan M, Liu K (2020) Rational design on separators and liquid electrolytes for safer lithium-ion batteries. J Energy Chem 43:58–70. https://doi.org/10.1016/j.jechem.2019.08.008
Zhao WM, Ji YJ, Zhang ZR, Lin M, Wu Z, Zheng X, Li Q, Yang Y (2017) Recent advances in the research of functional electrolyte additives for lithium-ion batteries. Curr Opin Electrochem 6(1):84–91. https://doi.org/10.1016/j.coelec.2017.10.012
Achiha T, Nakajima T, Ohzawa Y, Koh M, Yamauchi A, Kagawa M, Aoyama H (2010) Thermal Stability and electrochemical properties of fluorine compounds as nonflammable solvents for lithium-ion batteries. J Electrochem Soc 157(6):A707–A712. https://doi.org/10.1149/1.3377084
He MN, Hu LB, Xue Z, Su CC, Redfern P, Curtiss LA, Polzin B, von Cresce A, Xu R, Zhang ZC (2015) Fluorinated Electrolytes for 5-V Li-ion chemistry: probing voltage stability of electrolytes with electrochemical floating test. J Electrochem Soc 162(9):A1725–A1729. https://doi.org/10.1149/2.0231509jes
Zheng X, Liao Y, Zhang ZR, Zhu JP, Ren FC, He HJ, Xiang YX, Zheng YZ, Yang Y (2020) Exploring high-voltage fluorinated carbonate electrolytes for LiNi0.5Mn1.5O4 cathode in Li-ion batteries. J Energy Chem 42:62–70. https://doi.org/10.1016/j.jechem.2019.05.023
Zheng H, Zhou X, Cheng S, Xia R, Nie SP, Liang X, Sun Y, Xiang HF (2019) High-voltage LiNi0.5Mn1.5O4 cathode stability of fluorinated ether based on enhanced separator wettability. J Electrochem Soc 166(8):A1456–A1462. https://doi.org/10.1149/2.0601908jes
Hu L, Zhang Z, Amine K (2013) Fluorinated electrolytes for Li-ion battery: an FEC-based electrolyte for high voltage LiNi0.5Mn1.5O4/graphite couple. Electrochem Commun 35:76–79. https://doi.org/10.1016/j.elecom.2013.08.009
Hu LB, Amine K, Zhang ZC (2014) Fluorinated electrolytes for 5-V Li-ion chemistry: dramatic enhancement of LiNi0.5Mn1.5O4/graphite cell performance by a lithium reservoir. Electrochem Commun 44:34–37. https://doi.org/10.1016/j.elecom.2014.04.006
Zhang ZC, Hu LB, Wu HM, Weng W, Koh M, Redfern PC, Curtiss LA, Amine K (2013) Fluorinated electrolytes for 5 V lithium-ion battery chemistry. Energy Environ Sci 6(6):1806–1810. https://doi.org/10.1039/c3ee24414h
Luo Y, Lu T, Zhang Y, Yan L, Xie J, Mao SS (2016) Enhanced electrochemical performance of LiNi0.5Mn1.5O4 cathode using an electrolyte with 3-(1,1,2,2-tetrafluoroethoxy)-1,1,2,2-tetrafluoropropane. J Power Sources 323:134–141. https://doi.org/10.1016/j.jpowsour.2016.05.053
Lavi O, Luski S, Shpigel N, Menachem C, Pomerantz Z, Elias Y, Aurbach D (2020) Electrolyte solutions for rechargeable Li-ion batteries based on fluorinated solvents. ACS Appl Energy Mater 3(8):7485–7499. https://doi.org/10.1021/acsaem.0c00898
Schmidt M, Heider U, Kuehner A, Oesten R, Jungnitz M, Ignat’ev N, Sartori P (2001) Lithium fluoroalkylphosphates: a new class of conducting salts for electrolytes for high energy lithium-ion batteries. J Power Sources 97-98:557–560. https://doi.org/10.1016/S0378-7753(01)00640-1
Xu K (2004) Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem Rev 104(10):4303–4418. https://doi.org/10.1021/cr030203g
Xia Y, Hideshima Y, Kumada N, Nagano M, Yoshio M (1998) Studies on Li–Mn–O spinel system (obtained from melt-impregnation method) as a cathode for 4 V lithium batteries: Part V. Enhancement of the elevated temperature performance of Li/LiMn2O4 cells. J Power Sources 74(1):24–28. https://doi.org/10.1016/S0378-7753(98)00005-6
Im J, Lee J, Ryou M-H, Lee YM, Cho KY (2017) Fluorinated carbonate-based electrolyte for high-voltage Li(Ni0.5Mn0.3Co0.2)O2/graphite lithium-ion battery. J Electrochem Soc 164(1):A6381–A6385. https://doi.org/10.1149/2.0591701jes
Li Y, Veith GM, Browning KL, Chen J, Hensley DK, Paranthaman MP, Dai S, Sun X-G (2017) Lithium malonatoborate additives enabled stable cycling of 5 V lithium metal and lithium ion batteries. Nano Energy 40:9–19. https://doi.org/10.1016/j.nanoen.2017.07.051
Verdier S, El Ouatani L, Dedryvere R, Bonhomme F, Biensan P, Gonbeau D (2007) XPS study on Al2O3- and AlPO4-coated LiCoO2 cathode material for high-capacity li ion batteries. J Electrochem Soc 154(12):A1088–A1099. https://doi.org/10.1149/1.2789299
Acknowledgments
We acknowledge the support from the open fund of Fujian Provincial Key Laboratory of Functional Materials and Applications (Xiamen University of Technology, Grant No. fma2018008), the National Natural Science Foundation of China (Grants No. 21875196 and 21573184), and the Science and Technology Planning Projects of Fujian Province, China (Grant 2019H0003).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Z., Zhuang, S., Lu, M. et al. Exploring 1,1,2,2-tetrafluoroethyl-2,2,3,3-tetrafluoropropyl ether as a high voltage electrolyte solvent for 5-V Li2CoPO4F cathode. J Solid State Electrochem 25, 1353–1360 (2021). https://doi.org/10.1007/s10008-021-04915-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-021-04915-z