Abstract
Objectives
N-Acetyl cysteine (NAC), a well-known antioxidant molecule, has been used to modulate oxidative stress and inflammation. However, no studies have examined the effect of NAC in regenerative endodontic procedures (REPs). Therefore, the aim of this study was to investigate the effects of NAC on cell survival, mitochondrial reactive oxygen species (mtROS) production, and inflammatory and mitochondria-related gene expression on lipopolysaccharide (LPS)-treated apical papilla cells (APCs).
Materials and methods
To assess the NAC concentration, 5 and 10 mM NAC were administered to LPS-treated APCs. Cell proliferation was measured at 24, 48, and 72 h by using AlamarBlue® assay. The 5-mM concentration was further analyzed using different treatment durations: 10 min, 24 h, and the entire study period. The mtROS production was quantified using MitoSOX™ Red and MitoTracker™ Green. RT-PCR was used to detect the expression of IL-6 and TNF-α inflammatory genes and mitochondrial morphology–related genes (Mfn-2/Drp-1 and Bcl-2/Bax) at 6 and 24 h. The statistical significance level was set at 0.05.
Results
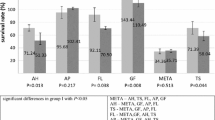
Five-millimolar NAC promoted the highest LPS-treated APC proliferation. The use of 24-h NAC stimulated cell proliferation, whereas the entire-period NAC application (> 48 h) significantly reduced the cell number. The mtROS levels were slightly altered after NAC induction. Ten-minute NAC treatment downregulated the IL-6 and TNF-α expression, whereas the expression of Bcl-2/Bax and Mfn-2/Drp-1 ratios was upregulated at 6 h.
Conclusions
Under the LPS-induced inflammatory condition, NAC stimulated APC survival and decreased inflammation. Ten-minute NAC treatment was sufficient to reduce the level of inflammation and maintain the mitochondrial dynamics.
Clinical relevance
Ten-minute NAC application is sufficient to reduce the level of inflammation and maintain the mitochondrial dynamics. Therefore, NAC may be considered as a potential adjunctive irrigation solution in REPs.




Similar content being viewed by others
References
Kahler B, Lin LM (2017) A review of regenerative endodontics: current protocols and future directions. J Istanbul Univ Fac Dent 51(3 Suppl 1):41–51
Iwaya SI, Ikawa M, Kubota M (2001) Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent Traumatol 17(4):185–187
Chandki R, Kala M, Banthia P, Banthia R (2012) From stem to roots: tissue engineering in endodontics. J Clin Exp Dent 4(1):e66–e71. https://doi.org/10.4317/jced.50678
Rafter M (2005) Apexification: a review. Dent Traumatol 21(1):1–8. https://doi.org/10.1111/j.1600-9657.2004.00284.x
Banchs F, Trope M (2004) Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod 30(4):196–200. https://doi.org/10.1097/00004770-200404000-00003
AAE clinical considerations for a regenerative procedure revised 4/1/2018 (2018) https://www.aae.org/specialty/wpcontent/uploads/sites/2/2018/06/ConsiderationsForRegEndo_AsOfApril2018.pdf. Accessed 26 Jun 2019
Nosrat A, Homayounfar N, Oloomi K (2012) Drawbacks and unfavorable outcomes of regenerative endodontic treatments of necrotic immature teeth: a literature review and report of a case. J Endod 38(10):1428–1434. https://doi.org/10.1016/j.joen.2012.06.025
Lovelace TW, Henry MA, Hargreaves KM, Diogenes A (2011) Evaluation of the delivery of mesenchymal stem cells into the root canal space of necrotic immature teeth after clinical regenerative endodontic procedure. J Endod 37(2):133–138
Huang GT, Sonoyama W, Liu Y, Liu H, Wang S, Shi S (2008) The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod 34(6):645–651. https://doi.org/10.1016/j.joen.2008.03.001
Dioguardi M, Di Gioia G, Illuzzi G, Arena C, Caponio VCA, Caloro GA, Zhurakivska K, Adipietro I, Troiano G, Lo Muzio L (2019) Inspection of the microbiota in endodontic lesions. Dent J (Basel) 7(2). https://doi.org/10.3390/dj7020047
Kim DY, Jun JH, Lee HL, Woo KM, Ryoo HM, Kim GS, Baek JH, Han SB (2007) N-acetylcysteine prevents LPS-induced pro-inflammatory cytokines and MMP2 production in gingival fibroblasts. Arch Pharm Res 30(10):1283–1292. https://doi.org/10.1007/bf02980269
Zhang J, Zhang Y, Lv H, Yu Q, Zhou Z, Zhu Q, Wang Z, Cooper PR, Smith AJ, Niu Z, He W (2013) Human stem cells from the apical papilla response to bacterial lipopolysaccharide exposure and anti-inflammatory effects of nuclear factor I C. J Endod 39(11):1416–1422. https://doi.org/10.1016/j.joen.2013.07.018
Li X, Wang X, Zheng M, Luan QX (2016) Mitochondrial reactive oxygen species mediate the lipopolysaccharide-induced pro-inflammatory response in human gingival fibroblasts. Exp Cell Res 347(1):212–221. https://doi.org/10.1016/j.yexcr.2016.08.007
Bullon P, Cordero MD, Quiles JL, Morillo JM, del Carmen Ramirez-Tortosa M, Battino M (2011) Mitochondrial dysfunction promoted by Porphyromonas gingivalis lipopolysaccharide as a possible link between cardiovascular disease and periodontitis. Free Radic Biol Med 50(10):1336–1343. https://doi.org/10.1016/j.freeradbiomed.2011.02.018
Katoh M, Wu B, Nguyen HB, Thai TQ, Yamasaki R, Lu H, Rietsch AM, Zorlu MM, Shinozaki Y, Saitoh Y, Saitoh S, Sakoh T, Ikenaka K, Koizumi S, Ransohoff RM, Ohno N (2017) Polymorphic regulation of mitochondrial fission and fusion modifies phenotypes of microglia in neuroinflammation. Sci Rep 7(1):4942. https://doi.org/10.1038/s41598-017-05232-0
He Y, Gan X, Zhang L, Liu B, Zhu Z, Li T, Zhu J, Chen J, Yu H (2018) CoCl2 induces apoptosis via a ROS-dependent pathway and Drp1-mediated mitochondria fission in periodontal ligament stem cells. Am J Phys Cell Phys 315(3):C389–C397. https://doi.org/10.1152/ajpcell.00248.2017
Chiong M, Cartes-Saavedra B, Norambuena-Soto I, Mondaca-Ruff D, Morales PE, Garcia-Miguel M, Mellado R (2014) Mitochondrial metabolism and the control of vascular smooth muscle cell proliferation. Front Cell Dev Biol 2:72. https://doi.org/10.3389/fcell.2014.00072
Berniakovich I, Laricchia-Robbio L, Izpisua Belmonte JC (2012) N-acetylcysteine protects induced pluripotent stem cells from in vitro stress: impact on differentiation outcome. Int J Dev Biol 56(9):729–735. https://doi.org/10.1387/ijdb.120070ji
Paranjpe A, Cacalano NA, Hume WR, Jewett A (2007) N-acetylcysteine protects dental pulp stromal cells from HEMA-induced apoptosis by inducing differentiation of the cells. Free Radic Biol Med 43(10):1394–1408. https://doi.org/10.1016/j.freeradbiomed.2007.07.011
Ulusoy AT, Kalyoncuoglu E, Reis A, Cehreli ZC (2016) Antibacterial effect of N-acetylcysteine and taurolidine on planktonic and biofilm forms of Enterococcus faecalis. Dent Traumatol 32(3):212–218. https://doi.org/10.1111/edt.12237
Camargo CHR, Gomes LCL, Franca MCM, Bittencourt TS, Valera MC, Camargo SEA, Bottino MC (2019) Incorporating N-acetylcysteine and tricalcium phosphate into epoxy resin-based sealer improved its biocompatibility and adhesiveness to radicular dentine. Dent Mater 35(12):1750–1756. https://doi.org/10.1016/j.dental.2019.09.001
Edlundh-Rose E, Kupershmidt I, Gustafsson AC, Parasassi T, Serafino A, Bracci-Laudiero L, Greco G, Krasnowska EK, Romano MC, Lundeberg T, Nilsson P, Lundeberg J (2005) Gene expression analysis of human epidermal keratinocytes after N-acetyl L-cysteine treatment demonstrates cell cycle arrest and increased differentiation. Pathobiology 72(4):203–212. https://doi.org/10.1159/000086790
Bhasin P, Sharma M, Bindal D, Tomar D, Sarin A, Sharma N (2019) An in vitro evaluation of antimicrobial effects of three different root canal irrigating solutions against Enterococcus faecalis and Streptococcus mutans. J Contemp Dent Pract 20(2):221–225
Zheng R, Tan Y, Gu M, Kang T, Zhang H, Guo L (2019) N-acetyl cysteine inhibits lipopolysaccharide-mediated synthesis of interleukin-1beta and tumor necrosis factor-alpha in human periodontal ligament fibroblast cells through nuclear factor-kappa B signaling. Medicine (Baltimore) 98(40):e17126. https://doi.org/10.1097/MD.0000000000017126
Choi YS, Kim C, Moon JH, Lee JY (2018) Removal and killing of multispecies endodontic biofilms by N-acetylcysteine. Braz J Microbiol 49(1):184–188. https://doi.org/10.1016/j.bjm.2017.04.003
Minamikawa H, Yamada M, Deyama Y, Suzuki K, Kaga M, Yawaka Y, Ogawa T (2011) Effect of N-acetylcysteine on rat dental pulp cells cultured on mineral trioxide aggregate. J Endod 37(5):637–641. https://doi.org/10.1016/j.joen.2011.02.012
Kim NR, Park HC, Kim I, Lim B-S, Yang H-C (2010) In vitro cytocompatibility of N-acetylcysteine–supplemented dentin bonding agents. J Endod 36(11):1844–1850
Jiao Y, Ma S, Wang Y, Li J, Shan L, Liu Q, Liu Y, Song Q, Yu F, Yu H, Liu H, Huang L, Chen J (2016) N-acetyl cysteine depletes reactive oxygen species and prevents dental monomer-induced intrinsic mitochondrial apoptosis in vitro in human dental pulp cells. PLoS One 11(1):e0147858. https://doi.org/10.1371/journal.pone.0147858
Chang M-C, Lin L-D, Wu M-T, Chan C-P, Chang H-H, Lee M-S, Sun T-Y, Jeng P-Y, Yeung S-Y, Lin H-J (2015) Effects of camphorquinone on cytotoxicity, cell cycle regulation and prostaglandin E2 production of dental pulp cells: role of ROS, ATM/Chk2, MEK/ERK and hemeoxygenase-1. PLoS One 10(12):e0143663
Lee DH, Lim B-S, Lee Y-K, Yang H-C (2007) Mechanisms of root canal sealers cytotoxicity on osteoblastic cell line MC3T3-E1. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 104(5):717–721
Xiong L, Sun J, Hirche C, Yang J, Yang Y, Xia Y, Lehnhardt M, Wang R, Fu X (2012) In vitro N-acetyl-L-cysteine promotes proliferation and suppresses interleukin-8 expression in adipose-derived stem cells. Aesthet Plast Surg 36(5):1260–1265. https://doi.org/10.1007/s00266-012-9960-8
Sun LY, Pang CY, Li DK, Liao CH, Huang WC, Wu CC, Chou YY, Li WW, Chen SY, Liu HW, Chang YJ, Cheng CF (2013) Antioxidants cause rapid expansion of human adipose-derived mesenchymal stem cells via CDK and CDK inhibitor regulation. J Biomed Sci 20:53. https://doi.org/10.1186/1423-0127-20-53
Ali F, Qadir AUR, Fatima N, Wajid N (2017) The effect of N-acetyl cysteine on H2O2 mediated oxidative stress in Whartonʼs jelly derived mesenchymal stem cells. Adv Life Sci 4(4):137–142
Debeljak Martacic J, Borozan S, Radovanovic A, Popadic D, Mojsilovic S, Vucic V, Todorovic V, Kovacevic Filipovic M (2016) N-acetyl-L-cysteine enhances ex-vivo amplification of deciduous teeth dental pulp stem cells. Arch Oral Biol 70:32–38. https://doi.org/10.1016/j.archoralbio.2016.06.002
Ali F, Rafique H, Wajid N (2015) N-acetylcysteine prevents cord derived stem cells from H2O2 induced injury in vitro. EJPMR 2:589–598
Kim E-C, Kim M-K, Leesungbok R, Lee S-W, Ahn S-J (2016) Co–Cr dental alloys induces cytotoxicity and inflammatory responses via activation of Nrf2/antioxidant signaling pathways in human gingival fibroblasts and osteoblasts. Dent Mater 32(11):1394–1405
Guo L, Zhang H, Li W, Zhan D, Wang M (2018) N-acetyl cysteine inhibits lipopolysaccharide-mediated induction of interleukin-6 synthesis in MC3T3-E1 cells through the NF-kB signaling pathway. Arch Oral Biol 93:149–154. https://doi.org/10.1016/j.archoralbio.2018.06.007
Pei Y, Liu H, Yang Y, Yang Y, Jiao Y, Tay FR, Chen J (2018) Biological activities and potential oral applications of n-acetylcysteine: progress and prospects. Oxid Med Cell Longev 2018:2835787. https://doi.org/10.1155/2018/2835787
Kim JC, Lee YH, Yu MK, Lee NH, Park JD, Bhattarai G, Yi HK (2012) Anti-inflammatory mechanism of PPARgamma on LPS-induced pulp cells: role of the ROS removal activity. Arch Oral Biol 57(4):392–400. https://doi.org/10.1016/j.archoralbio.2011.09.009
Golz L, Memmert S, Rath-Deschner B, Jager A, Appel T, Baumgarten G, Gotz W, Frede S (2014) LPS from P. gingivalis and hypoxia increases oxidative stress in periodontal ligament fibroblasts and contributes to periodontitis. Mediat Inflamm 2014:986264–986213. https://doi.org/10.1155/2014/986264
Cheng R, Choudhury D, Liu C, Billet S, Hu T, Bhowmick NA (2015) Gingival fibroblasts resist apoptosis in response to oxidative stress in a model of periodontal diseases. Cell Death Dis 1:15046. https://doi.org/10.1038/cddiscovery.2015.46
Karadzic I, Vucic V, Jokanovic V, Debeljak-Martacic J, Markovic D, Petrovic S, Glibetic M (2015) Effects of novel hydroxyapatite-based 3D biomaterials on proliferation and osteoblastic differentiation of mesenchymal stem cells. J Biomed Mater Res A 103(1):350–357. https://doi.org/10.1002/jbm.a.35180
Xiao Y, Li X, Cui Y, Zhang J, Liu L, Xie X, Hao H, He G, Kander MC, Chen M, Liu Z, Verfaillie CM, Zhu H, Lei M, Liu Z (2014) Hydrogen peroxide inhibits proliferation and endothelial differentiation of bone marrow stem cells partially via reactive oxygen species generation. Life Sci 112(1–2):33–40. https://doi.org/10.1016/j.lfs.2014.07.016
Martacic J, Filipovic MK, Borozan S, Cvetkovic Z, Popovic T, Arsic A, Takic M, Vucic V, Glibetic M (2018) N-acetyl-L-cysteine protects dental tissue stem cells against oxidative stress in vitro. Clin Oral Investig 22(8):2897–2903. https://doi.org/10.1007/s00784-018-2377-2
Diomede F, Marconi GD, Guarnieri S, D'Attilio M, Cavalcanti M, Mariggio MA, Pizzicannella J, Trubiani O (2019) A novel role of ascorbic acid in anti-inflammatory pathway and ROS generation in HEMA treated dental pulp stem cells. Materials (Basel) 13(1). https://doi.org/10.3390/ma13010130
Zhang Y, Xiao J-f, Yang H-f, Cao W-w, Shi H-m, Cun J-f, Tay FR, Ping J, Jiao Y, Xiao Y-h (2020) N-acetyl cysteine as a novel polymethyl methacrylate resin component: protection against cell apoptosis and genotoxicity. Oxid Med Cell Longev 2020:1301736. https://doi.org/10.1155/2019/1301736
Jiao Y, Ma S, Li J, Shan L, Wang Y, Tian M, Yang Y, Sun J, Ban J, Chen J (2015) N-acetyl cysteine (NAC)-directed detoxification of methacryloxylethyl cetyl ammonium chloride (DMAE-CB). PLoS One 10(8):e0135815. https://doi.org/10.1371/journal.pone.0135815
Sato N, Ueno T, Kubo K, Suzuki T, Tsukimura N, Att W, Yamada M, Hori N, Maeda H, Ogawa T (2009) N-acetyl cysteine (NAC) inhibits proliferation, collagen gene transcription, and redox stress in rat palatal mucosal cells. Dent Mater 25(12):1532–1540. https://doi.org/10.1016/j.dental.2009.07.006
Valdivieso AG, Dugour AV, Sotomayor V, Clauzure M, Figueroa JM, Santa-Coloma TA (2018) N-acetyl cysteine reverts the proinflammatory state induced by cigarette smoke extract in lung Calu-3 cells. Redox Biol 16:294–302. https://doi.org/10.1016/j.redox.2018.03.006
Cheng G, Zielonka M, Dranka B, Kumar SN, Myers CR, Bennett B, Garces AM, Dias Duarte Machado LG, Thiebaut D, Ouari O, Hardy M, Zielonka J, Kalyanaraman B (2018) Detection of mitochondria-generated reactive oxygen species in cells using multiple probes and methods: potentials, pitfalls, and the future. J Biol Chem 293(26):10363–10380. https://doi.org/10.1074/jbc.RA118.003044
Ohnishi T, Bandow K, Kakimoto K, Kusuyama J, Matsuguchi T (2014) Long-time treatment by low-dose N-acetyl-L-cysteine enhances proinflammatory cytokine expressions in LPS-stimulated macrophages. PLoS One 9(2):e87229. https://doi.org/10.1371/journal.pone.0087229
Jun J-H, Lee H-L, Woo KM, Ryoo H-M, Kim G-S, Baek J-H, Han S-B (2007) N-acetyicysteine prevents lps-lnduced pro-inflammatory cytokines and mmp2 production in gingival fibroblasts. Arch Pharm Res 30(10):1283
Manicone AM, McGuire JK (2008) Matrix metalloproteinases as modulators of inflammation. In: Seminars in cell & developmental biology, vol 1. Elsevier, pp 34–41
Lozhkin A, Vendrov AE, Pan H, Wickline SA, Madamanchi NR, Runge MS (2017) NADPH oxidase 4 regulates vascular inflammation in aging and atherosclerosis. J Mol Cell Cardiol 102:10–21. https://doi.org/10.1016/j.yjmcc.2016.12.004
Ricciotti E, FitzGerald GA (2011) Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 31(5):986–1000. https://doi.org/10.1161/ATVBAHA.110.207449
Ren K, Torres R (2009) Role of interleukin-1beta during pain and inflammation. Brain Res Rev 60(1):57–64. https://doi.org/10.1016/j.brainresrev.2008.12.020
Yang H, Zhu YT, Cheng R, Shao MY, Fu ZS, Cheng L, Wang FM, Hu T (2010) Lipopolysaccharide-induced dental pulp cell apoptosis and the expression of Bax and Bcl-2 in vitro. Braz J Med Biol Res 43(11):1027–1033. https://doi.org/10.1590/s0100-879x2010007500102
Renault TT, Manon S (2011) Bax: addressed to kill. Biochimie 93(9):1379–1391. https://doi.org/10.1016/j.biochi.2011.05.013
Chand HS, Harris JF, Tesfaigzi Y (2018) IL-13 in LPS-induced inflammation causes Bcl-2 expression to sustain hyperplastic mucous cells. Sci Rep 8(1):436. https://doi.org/10.1038/s41598-017-18884-9
Fu W, Liu Y, Yin H (2019) Mitochondrial dynamics: biogenesis, fission, fusion, and mitophagy in the regulation of stem cell behaviors. Stem Cells Int 2019:9757201–9757215. https://doi.org/10.1155/2019/9757201
Givvimani S, Pushpakumar S, Veeranki S, Tyagi SC (2014) Dysregulation of Mfn2 and Drp-1 proteins in heart failure. Can J Physiol Pharmacol 92(7):583–591. https://doi.org/10.1139/cjpp-2014-0060
Lee JE, Seo BJ, Han MJ, Hong YJ, Hong K, Song H, Lee JW, Do JT (2020) Changes in the expression of mitochondrial morphology-related genes during the differentiation of murine embryonic stem cells. Stem Cells Int 2020:1–12
Acknowledgments
The authors thank the Center of Molecular and Cell Biology of Infectious Diseases Research and Cardiac Electrophysiology Research and Training Center, Chiang Mai University, for the provision of equipment. They also thank Dr. M. Kevin O Carroll, Professor Emeritus of the University of Mississippi School of Dentistry, Oxford, MS, and Faculty Consultant at Chiang Mai University, for his assistance in the preparation of the manuscript.
Funding
The work was supported by a grant from the Faculty of Dentistry of Chiang Mai University.
Author information
Authors and Affiliations
Contributions
Nutcha Jariyamana: validation, formal analysis, investigation, writing—original draft, visualization. Patchanee Chuveera: resources. Anat Dewi: data curation. Warat Leelapornpisid: data curation. Jitjiroj Ittichaicharoen: resources. Siriporn Chattipakorn: supervision. Tanida Srisuwan: conceptualization, methodology, writing—review and editing, project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards by the Human Experimentation Committee, Faculty of Dentistry, Chiang Mai University (No. 13/2019) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jariyamana, N., Chuveera, P., Dewi, A. et al. Effects of N-acetyl cysteine on mitochondrial ROS, mitochondrial dynamics, and inflammation on lipopolysaccharide-treated human apical papilla cells. Clin Oral Invest 25, 3919–3928 (2021). https://doi.org/10.1007/s00784-020-03721-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-020-03721-7