Abstract
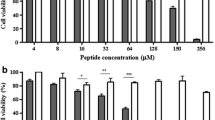
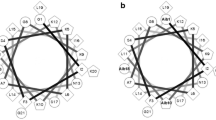
Alyteserin-2a (ILGKLLSTAAGLLSNL.NH2) is a cationic, amphipathic α-helical cell-penetrating peptide, first isolated from skin secretions of the midwife toad Alytes obstetricans. Structure–activity relationships were investigated by synthesizing analogs of alyteserin-2a in which amino acids on the hydrophobic face of the helix were replaced by l-tryptophan and amino acids on the hydrophilic face were replaced by one or more l-lysine or d-lysine residues. The Trp-containing peptides display increased cytotoxic activity against non-small cell lung adenocarcinoma A549 cells (up to 11-fold), but hemolytic activity against human erythrocytes increases in parallel. The potency of the N15K analog against A549 cells (LC50 = 13 μM) increases sixfold relative to alyteserin-2a and the therapeutic index (ratio of LC50 for erythrocytes and tumor cells) increases twofold. Incorporation of a d-Lys11 residue into the N15K analog generates a peptide that retains potency against A549 cells (LC50 = 15 μM) but whose therapeutic index is 13-fold elevated relative to the native peptide. [G11k, N15K] alyteserin-2a is also active against human hepatocarcinoma HepG2 cells (LC50 = 26 μM), breast adenocarcinoma MDA-MB-231 cells (LC50 = 20 μM), and colorectal adenocarcinoma HT-29 cells (LC50 = 28 μM). [G11k, N15K] alyteserin-2a, in concentrations as low as 1 μg/mL, significantly (P < 0.05) inhibits the release of the immune-suppressive cytokines IL-10 and TGF-β from unstimulated and concanavalin A-stimulated peripheral blood mononuclear cells. The data suggest a strategy of increasing the cationicity while reducing the helicity of naturally occurring amphipathic α-helical peptides to generate analogs with improved cytotoxicity against tumor cells but decreased activity against non-neoplastic cells.



Similar content being viewed by others
References
Almeida PF, Pokorny A (2009) Mechanisms of antimicrobial, cytolytic, and cell-penetrating peptides: from kinetics to thermodynamics. Biochemistry 48:8083–8093
Bocchinfuso G, Palleschi A, Orioni B, Grande G, Formaggio F, Toniolo C, Park Y, Hahm KS, Stella L (2009) Different mechanisms of action of antimicrobial peptides: insights from fluorescence spectroscopy experiments and molecular dynamics simulations. J Pept Sci 15:550–558
Calone I, Souchelnytskyi S (2012) Inhibition of TGFβ signaling and its implications in anticancer treatments. Exp Oncol 34:9–16
Chen ZS, Tiwari AK (2011) Multidrug resistance proteins (MRPs/ABCCs) in cancer chemotherapy and genetic diseases. FEBS J 278:3226–3245
Chen Y, Mant CT, Farmer SW, Hancock RE, Vasil ML, Hodges RS (2005) Rational design of alpha-helical antimicrobial peptides with enhanced activities and specificity/therapeutic index. J Biol Chem 280:12316–21229
Chen Y, Guarnieri MT, Vasil AI, Vasil ML, Mant CT, Hodges RS (2007) Role of peptide hydrophobicity in the mechanism of action of alpha-helical antimicrobial peptides. Antimicrob Agents Chemother 51:1398–1406
Conlon JM (2011a) The contribution of skin antimicrobial peptides to the system of innate immunity in anurans. Cell Tissue Res 343:201–212
Conlon JM (2011b) Structural diversity and species distribution of host-defense peptides in frog skin secretions. Cell Mol Life Sci 68:2303–2315
Conlon JM (2012) The potential of frog skin antimicrobial peptides for development into therapeutically valuable anti-infective agents. In: Rajasekaran K, Cary JW, Jaynes J, Montesinos E (eds) Small wonders: peptides for disease control. American chemical society symposium series, Washington, DC, pp 47–60
Conlon JM, Al-Ghaferi N, Abraham B, Leprince J (2007a) Strategies for transformation of naturally-occurring amphibian antimicrobial peptides into therapeutically valuable anti-infective agents. Methods 42:349–357
Conlon JM, Woodhams DC, Raza H, Coquet L, Leprince J, Jouenne T, Vaudry H, Rollins-Smith LA (2007b) Peptides with differential cytolytic activity from skin secretions of the lemur leaf frog Hylomantis lemur (Hylidae: Phyllomedusinae). Toxicon 50:498–506
Conlon JM, Galadari S, Raza H, Condamine E (2008) Design of potent, non-toxic antimicrobial agents based upon the naturally occurring frog skin peptides, ascaphin-8 and peptide XT-7. Chem Biol Drug Des 72:58–64
Conlon JM, Demandt A, Nielsen PF, Leprince J, Vaudry H, Woodhams DC (2009) The alyteserins: two families of antimicrobial peptides from the skin secretions of the midwife toad Alytes obstetricans (Alytidae). Peptides 30:1069–1073
Conlon JM, Mechkarska M, Arafat K, Attoub S, Sonnevend A (2012) Analogues of the frog skin peptide alyteserin-2a with enhanced antimicrobial activities against Gram-negative bacteria. J Pept Sci 18:270–275
Cruciani RA, Barker JL, Zasloff M, Chen HC, Colamonici O (1991) Antibiotic magainins exert cytolytic activity against transformed cell lines through channel formation. Proc Natl Acad Sci USA 88:3792–3796
Dathe M, Wieprecht T (1999) Structural features of helical antimicrobial peptides: their potential to modulate activity on model membranes and biological cells. Biochim Biophys Acta 1462:71–87
Dathe M, Schümann M, Wieprecht T, Winkler A, Beyermann M, Krause E, Matsuzaki K, Murase O, Bienert M (1996) Peptide helicity and membrane surface charge modulate the balance of electrostatic and hydrophobic interactions with lipid bilayers and biological membranes. Biochemistry 35:12612–12622
Dathe M, Wieprecht T, Nikolenko H, Handel L, Maloy WL, MacDonald DL, Beyermann M, Bienert M (1997) Hydrophobicity, hydrophobic moment and angle subtended by charged residues modulate antibacterial and haemolytic activity of amphipathic helical peptides. FEBS Lett 403:208–212
Dobrzyńska I, Szachowicz-Petelska B, Sulkowski S, Figaszewski Z (2005) Changes in electric charge and phospholipids composition in human colorectal cancer cells. Mol Cell Biochem 276:113–119
Galizia G, Orditura M, Romano C, Lieto E, Castellano P, Pelosio L, Imperatore V, Catalano G, Pignatelli C, De Vita F (2002) Prognostic significance of circulating IL-10 and IL-6 serum levels in colon cancer patients undergoing surgery. Clin Immunol 102:169–178
Giangaspero A, Sandri L, Tossi A (2001) Amphipathic alpha helical antimicrobial peptides. Eur J Biochem 268:5589–5600
Hoskin DW, Ramamoorthy A (2008) Studies on anticancer activities of antimicrobial peptides. Biochim Biophys Acta 1778:357–375
Huang Y, Huang J, Chen Y (2010) Alpha-helical cationic antimicrobial peptides: relationships of structure and function. Protein Cell 1:143–152
Iozzo RV, Sanderson RD (2011) Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J Cell Mol Med 15:1013–1031
Kindrachuk J, Napper S (2010) Structure–activity relationships of multifunctional host defence peptides. Mini Rev Med Chem 10:596–614
Lehmann J, Retz M, Sidhu SS, Suttmann H, Sell M, Paulsen F, Harder J, Unteregger G, Stöckle M (2006) Antitumor activity of the antimicrobial peptide magainin II against bladder cancer cell lines. Eur Urol 50:141–147
Libério MS, Joanitti GA, Azevedo RB, Cilli EM, Zanotta LC, Nascimento AC, Sousa MV, PiresJúnior OR, Fontes W, Castro MS (2011) Anti-proliferative and cytotoxic activity of pentadactylin isolated from Leptodactylus labyrinthicus on melanoma cells. Amino Acids 40:51–59
Lu CX, Nan KJ, Lei Y (2008) Agents from amphibians with anticancer properties. Anticancer Drugs 19:931–939
McDermott KM, Crocker PR, Harris A, Burdick MD, Hinoda Y, Hayashi T, Imai K, Hollingsworth MA (2001) Overexpression of MUC1 reconfigures the binding properties of tumor cells. Int J Cancer 94:783–791
Muñoz V, Serrano L (1994) Elucidating the folding problem of helical peptides using empirical parameters. Nat Struct Biol 1:399–409
Ohsaki Y, Gazdar AF, Chen HC, Johnson BE (1992) Antitumor activity of magainin analogues against human lung cancer cell lines. Cancer Res 52:3534–3538
Pacor S, Giangaspero A, Bacac M, Sava G, Tossi A (2002) Analysis of the cytotoxicity of synthetic antimicrobial peptides on mouse leucocytes: implications for systemic use. J Antimicrob Chemother 50:339–348
Riedl S, Zweytick D, Lohner K (2011) Membrane-active host defense peptides—challenges and perspectives for the development of novel anticancer drugs. Chem Phys Lipids 164:766–781
Rolston KV, Bodey GP, Safdar A (2007) Polymicrobial infection in patients with cancer: an underappreciated and underreported entity. Clin Infect Dis 45:228–233
Rozek T, Wegener KL, Bowie JH, Olver IN, Carver JA, Wallace JC, Tyler MJ (2000) The antibiotic and anticancer active aurein peptides from the Australian Bell Frogs Litoria aurea and Litoria raniformis the solution structure of aurein 1.2. Eur J Biochem 267:5330–5341
Schiffer M, Edmundson AB (1967) Use of helical wheels to represent the structures of proteins and to identify segments with helical potential. Biophys J 7:121–135
Schweizer F (2009) Cationic amphiphilic peptides with cancer-selective toxicity. Eur J Pharmacol 625:190–194
Sredni B (2012) Immunomodulating tellurium compounds as anti-cancer agents. Semin Cancer Biol 22:60–69
Srivastava MK, Andersson Å, Zhu L, Harris-White M, Lee JM, Dubinett S, Sharma S (2012) Myeloid suppressor cells and immune modulation in lung cancer. Immunotherapy 4:291–304
Stark M, Liu LP, Deber CM (2002) Cationic hydrophobic peptides with antimicrobial activity. Antimicrob Agents Chemother 46:3585–3590
Tachi T, Epand RF, Epand RM, Matsuzaki K (2002) Position dependent hydrophobicity of the antimicrobial magainin peptide affects the mode of peptide–lipid interactions and selective toxicity. Biochemistry 41:10723–10731
van Zoggel H, Hamma-Kourbali Y, Galanth C, Ladram A, Nicolas P, Courty J, Amiche M, Delbé J (2012) Antitumor and angiostatic peptides from frog skin secretions. Amino Acids 42:385–395
Wang C, Li HB, Li S, Tian LL, Shang DJ (2012) Antitumor effects and cell selectivity of temporin-1CEa, an antimicrobial peptide from the skin secretions of the Chinese brown frog (Rana chensinensis). Biochimie 94:434–441
Wieprecht T, Dathe M, Beyermann M, Krause E, Maloy WL, MacDonald DL, Bienert M (1997) Peptide hydrophobicity controls the activity and selectivity of magainin 2 amide in interaction with membranes. Biochemistry 36:6124–6132
Wimley WC, White SH (1996) Experimentally determined hydrophobicity scale for proteins at membrane interfaces. Nat Struct Biol 13:842–848
Won HS, Seo MD, Jung SJ, Lee SJ, Kang SJ, Son WS, Kim HJ, Park TK, Park SJ, Lee BJ (2006) Structural determinants for the membrane interaction of novel bioactive undecapeptides derived from gaegurin 5. J Med Chem 49:4886–4895
Yeung AT, Gellatly SL, Hancock RE (2011) Multifunctional cationic host defence peptides and their clinical applications. Cell Mol Life Sci 68:2161–2176
Zaiou M (2007) Multifunctional antimicrobial peptides: therapeutic targets in several human diseases. J Mol Med 85:317–329
Acknowledgments
This work was supported by a University Grant (G00000900) and a Faculty Support Grant (NP-10-12/103) from the United Arab Emirates University and by a grant from the Terry Fox for Cancer Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Conlon, J.M., Mechkarska, M., Prajeep, M. et al. Transformation of the naturally occurring frog skin peptide, alyteserin-2a into a potent, non-toxic anti-cancer agent. Amino Acids 44, 715–723 (2013). https://doi.org/10.1007/s00726-012-1395-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-012-1395-7