Abstract
Dengue is a disease caused by a flavivirus that is transmitted principally by the bite of an Aedes aegypti mosquito and represents a major public-health problem. Many studies have been carried out to identify soluble factors that are involved in the pathogenesis of this infection. Cytokines, soluble factors, and oxidative stress have been reported to be involved in the development of severe disease. Angiotensin II (Ang II) is a hormone with the ability to induce the production of cytokines and soluble factors related to the inflammatory processes and coagulation disorders observed in dengue. However, a direct involvement of Ang II in this disease has not been demonstrated. This review primarily summarizes the pathophysiology of dengue, the role of Ang II in various diseases, and reports that are highly suggestive of the involvement of this hormone in dengue.





Similar content being viewed by others
References
Martina BE, Koraka P, Osterhaus AD (2009) Dengue virus pathogenesis: an integrated view. Clin Microbiol Rev 22:564–581. https://doi.org/10.11128/CMR.00035-09
Dandona P, Dhindsa S, Ghanim H, Chaudhuri A (2007) Angiotensin II and Inflammation: The Effect of Angiotensin-Converting Enzyme Inhibition and Angiotensin II Receptor Blockade. J Hum Hypertens 21:20–27. https://doi.org/10.1038/sj.jhh.1002101
Timmermans PB, Wong PC, Chiu AT, Herblin WF, Benfield P, Carini DJ, Lee RJ, Wexler RR, Saye JA, Smith RD (1993) Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev 45:205–251
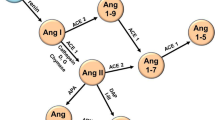
Ferrario CM, Chappell MC (2004) Novel angiotensin peptides. Cell Mol Life Sci 61:2720–2727. https://doi.org/10.1007/s00018-004-4243-4
Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos AJ, da Costa J, Zhang L, Pei Y, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, Backx PH, Yagil Y, Penninger JM (2002) Angiotensin converting enzyme 2 is an essential regulator of heart function. Nature 417:822–828. https://doi.org/10.1038/nature00786
Reaux A, Fournie-Zaluski MC, Llorens-Cortes C (2001) Angiotensin III: a central regulator of vasopressin release and blood pressure. Trends Endocrinol Metab 12:157–162. https://doi.org/10.1016/s1043-2760(01)00381-2
Hunyady L, Catt KJ (2006) Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol Endocrinol 20:953–970. https://doi.org/10.1210/me.2004-0536
Watanabe Y, Nagai Y, Takatsu K (2013) Activation and regulation of the pattern recognition receptors in obesity-induced adipose tissue inflammation and insulin resistance. Nutrients 5:3757–3778. https://doi.org/10.3390/nu5093757
Ryder E, Pedreañez A, Vargas R, Peña C, Fernandez E, Diez-Ewald M, Mosquera J (2015) Increased proinflammatory markers and lipoperoxidation in obese individuals: Inicial inflammatory events? Diabetes Metab Syndr 9:280–286. https://doi.org/10.1016/j.dsx.2014.04.022
Vargas R, Diez-Ewald M, Mosquera J, Durán A, Valero N, Pedreañez A, Peña C, Fernández E (2016) Increased C-reactive protein and decreased Interleukin-2 content in serum from obese individuals with or without insulin resistance: Associations with leukocyte count and insulin and adiponectin content. Diabetes Metab Syndr 10:S34–41. https://doi.org/10.1016/j.dsx.2015.09.007
Zhou Y, Chi J, Lv W, Wang Y (2021) Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (Covid-19). Diabetes Metab Res Rev 37:e3377. https://doi.org/10.1002/dmrr.3377
Mosquera-Sulbaran J, Pedreañez A, Carrero Y, Callejas D (2021) C-reactive protein as an effector molecule in the COVID-19 pathogenesis. Rev Med Virol 1–8:e2221. https://doi.org/10.1002/rmv.2221
Malavige GN, Chandima Jeewandara C, Graham S, Ogg GS (2022) Dengue and COVID-19: two sides of the same coin. J Biomed Sci 29:48. https://doi.org/10.1186/s12929-022-00833-y
Henrina J, Putra ICS, Lawrensia S, Handoyono QF, Cahyadi ASN (2020) Coronavirus Disease of 2019: a Mimicker of Dengue Infection? Compr Clin Med 2:1109–1119. https://doi.org/10.1007/s42399-020-00364-3
Loe MWC, Lee RCH, Chu JJH (2019) Antiviral activity of the FDA-approved drug candesartan cilexetil against Zika virus infection. Antiviral Res 172:104637. https://doi.org/10.1016/j.antiviral.2019.104637
Hernández-Fonseca JP, Durán A, Valero N, Mosquera J (2015) Losartan and enalapril decrease viral absorption and interleukin 1 beta production by macrophages in an experimental dengue virus infection. Arch Virol 160:2861–2865. https://doi.org/10.1007/s00705-015-2581-1
Madaka F, Tossaton Charoonratana T (2018) Angiotensin-converting enzyme inhibitory activity of Senna garrettiana active compounds: Potential markers for standardized herbal medicines. Phcog Mag 14:335–339. https://doi.org/10.4103/pm.pm_325_17
Tissera H, Rathore APS, Leong WY, Pike BL, Warkentien TE, Farouk FS, Syenina A, Eong Ooi E, Gubler DJ, Wilder-Smith A, St John AL (2017) Chymase Level Is a Predictive Biomarker of Dengue Hemorrhagic Fever in Pediatric and Adult Patients. J Infect Dis 216:1112–1121. https://doi.org/10.1093/infdis/jix447
St John AL, Rathore AP, Raghavan B, Ng ML, Abraham SN (2013) Contributions of mast cells and vasoactive products, leukotrienes and chymase, to dengue virus-induced vascular leakage. Elife 2:e00481. https://doi.org/10.7554/eLife.00481
Guha-Sapir D, Schimmer B (2005) Dengue fever: new paradigms for a changing epidemiology. Emerg Themes Epidemiol 2:1. https://doi.org/10.1186/1742-7622-2-1
Harris E, Videa E, Perez L, Sandoval E, Tellez Y, Perez ML, Cuadra R, Rocha J, Idiaquez W, Alonso RE, Delgado MA, Campo LA, Acevedo F, Gonzalez A, Amador JJ, Balmaseda A (2000) Clinical, epidemiologic, and virologic features of dengue in the 1998 epidemic in Nicaragua. Am J Trop Med Hyg 63:5–11. https://doi.org/10.4269/ajtmh.2000.63.5
Carlos CC, Oishi K, Cinco MT, Mapua CA, Inoue S, Cruz DJ, Pancho MA, Tanig CZ, Matias RR, Morita K, Natividad FF, Igarashi A, Nagatake T (2005) Comparison of clinical features and hematologic abnormalities between dengue fever and dengue hemorrhagic fever among children in the Philippines. Am J Trop Med Hyg 73:435–440
Setiati TE, Mairuhu AT, Koraka P, Supriatna M, Mac Gillavry MR, Brandjes DP, Osterhaus AD, van der Meer JW, van Gorp EC, Soemantri A (2007) Dengue disease severity in Indonesian children: an evaluation of the World Health Organization classification system. BMC Infect Dis 7:22. https://doi.org/10.1186/1471-2334-7-22
World Health Organization (1997) Dengue haemorrhagic fever: diagnosis, treatment, prevention and control, 2nd edn. WHO, Geneva, Switzerland
Wu SJ, Grouard-Vogel G, Sun W, Mascola JR, Brachtel E, Putvatana R, Louder MK, Filgueira L, Marovich MA, Wong HK, Blauvelt A, Murphy GS, Robb ML, Innes BL, Birx DL, Hayes CG, Frankel SS (2000) Human skin Langerhans cells are targets of dengue virus infection. Nat Med 6:816–820. https://doi.org/10.1038/77553
Limon-Flores AY, Perez-Tapia M, Estrada-Garcia I, Vaughan G, Escobar-Gutierrez A, Calderon-Amador J, Herrera-Rodriguez SE, Brizuela- Garcia A, Heras-Chavarria M, Flores-Langarica A, Cedillo-Barron L, Flores-Romo L (2005) Dengue virus inoculation to human skin explants: an effective approach to assess in situ the early infection and the effects on cutaneous dendritic cells. Int J Exp Pathol 86:323–334. https://doi.org/10.1111/j.0959-9673.2005.00445.x
Boonnak K, Slike BM, Burgess TH, Mason RM, Wu SJ, Sun P, Porter K, Rudiman IF, Yuwono D, Puthavathana P, Marovich MA (2008) Role of dendritic cells in antibody-dependent enhancement of dengue virus infection. J Virol 82:3939–3951. https://doi.org/10.1128/JVI.02484-07
Ho LJ, Shaio MF, Chang DM, Liao CL, Lai JH (2004) Infection of human dendritic cells by dengue virus activates and primes T cells towards Th0-like phenotype producing both Th1 and Th2 cytokines. Immunol Investig 33:423–437. https://doi.org/10.1081/imm-200038680
Ho LJ, Wang JJ, Shaio MF, Kao CL, Chang DM, Han SW, Lai JH (2001) Infection of human dendritic cells by dengue virus causes cell maturation and cytokine production. J Immunol 166:1499–1506. https://doi.org/10.4049/jimmunol.166.3.1499
Kwan WH, Helt AM, Maranon C, Barbaroux JB, Hosmalin A, Harris E, Fridman WH, Mueller CG (2005) Dendritic cell precursors are permissive to dengue virus and human immunodeficiency virus infection. J Virol 79:7291–7299. https://doi.org/10.1128/JVI.79.12.7291-7299.2005
Libraty DH, Pichyangkul S, Ajariyakhajorn C, Endy TP, Ennis FA (2001) Human dendritic cells are activated by dengue virus infection: enhancement by gamma interferon and implications for disease pathogenesis. J Virol 75:3501–3508. https://doi.org/10.1128/JVI.75.8.3501-3508.2001
Blackley S, Kou Z, Chen H, Quinn M, Rose RC, Schlesinger JJ, Coppage M, Jin X (2007) Primary human splenic macrophages, but not T or B cells, are the principal target cells for dengue virus infection in vitro. J Virol 81:13325–13334. https://doi.org/10.1128/JVI.01568-07
de Macedo FC, Nicol AF, Cooper LD, Yearsley M, Pires AR, Nuovo GJ (2006) Histologic, viral, and molecular correlates of dengue fever infection of the liver using highly sensitive immunohistochemistry. Diagn Mol Pathol 15:223–228. https://doi.org/10.1097/01.pdm.0000213462.60645.cd
Huerre MR, Lan NT, Marianneau P, Hue NB, Khun H, Hung NT, Khen NT, Drouet MT, Huong VT, Ha DQ, Buisson Y, Deubel V (2001) Liver histopathology and biological correlates in five cases of fatal dengue fever in Vietnamese children. Virchows Arch 438:107–115. https://doi.org/10.1007/s004280000329
Jessie K, Fong MY, Devi S, Lam SK, Wong KT (2004) Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J Infect Dis 189:1411–1418. https://doi.org/10.1086/383043
Kou Z, Quinn M, Chen H, Rodrigo WW, Rose RC, Schlesinger JJ, Jin X (2008) Monocytes, but not T or B cells, are the principal target cells for dengue virus (DV) infection among human peripheral blood mononuclear cells. J Med Virol 80:134–146. https://doi.org/10.1002/jmv.21051
Bosch I, Xhaja K, Estevez L, Raines G, Melichar H, Warke RV, Fournier MV, Ennis FA, Rothman AL (2002) Increased production of interleukin-8 in primary human monocytes and in human epithelial and endothelial cell lines after dengue virus challenge. J Virol 76:5588–5597. https://doi.org/10.1128/jvi.76.11.5588-5597.2002
Chen YC, Wang SY (2002) Activation of terminally differentiated human monocytes/macrophages by dengue virus: productive infection, hierarchical production of innate cytokines and chemokines, and the synergistic effect of lipopolysaccharide. J Virol 76:9877–9887. https://doi.org/10.1128/jvi.76.19.9877-9887.2002
Chao YC, Huang CS, Lee CN, Chang SY, King CC, Kao CL (2008) Higher infection of dengue virus serotype 2 in human monocytes of patients with G6PD deficiency. PLoS ONE 3:e1557. https://doi.org/10.1371/journal.pone.0001557
Durbin AP, Vargas MJ, Wanionek K, Hammond SN, Gordon A, Rocha C, Balmaseda A, Harris E (2008) Phenotyping of peripheral blood mononuclear cells during acute dengue illness demonstrates infection and increased activation of monocytes in severe cases compared to classic dengue fever. Virology 376:429–435. https://doi.org/10.1016/j.virol.2008.03.028
Kangwanpong D, Bhamarapravati N, Lucia HL (1995) Diagnosing dengue virus infection in archived autopsy tissues by means of the in situ PCR method: a case report. Clin Diagn Virol 3:165–172. https://doi.org/10.1016/0928-0197(94)00032-p
Balsitis SJ, Coloma J, Castro G, Alava A, Flores D, McKerrow JH, Beatty PR, Harris E (2009) Tropism of dengue virus in mice and humans defined by viral nonstructural protein 3-specific immunostaining. Am J Trop Med Hyg 80:416–424
Couvelard A, Marianneau P, Bedel C, Drouet MT, Vachon F, Henin D, Deubel V (1999) Report of a fatal case of dengue infection with hepatitis: demonstration of dengue antigens in hepatocytes and liver apoptosis. Hum Pathol 30:1106–1110. https://doi.org/10.1016/s0046-8177(99)90230-7
Miagostovich MP, Ramos RG, Nicol AF, Nogueira RM, Cuzzi- Maya T, Oliveira AV, Marchevsky RS, Mesquita RP, Schatzmayr HG (1997) Retrospective study on dengue fatal cases. Clin Neuropathol 16:204–208
Sariol CA, Pelegrino JL, Martinez A, Arteaga E, Kouri G, Guzman MG (1999) Detection and genetic relationship of dengue virus sequences in seventeen-year-old paraffin-embedded samples from Cuba. Am J Trop Med Hyg 61:994–1000. https://doi.org/10.4269/ajtmh.1999.61.994
Basilio-de-Oliveira CA, Aguiar GR, Baldanza MS, Barth OM, Eyer-Silva WA, Paes MV (2005) Pathologic study of a fatal case of dengue-3 virus infection in Rio de Janeiro, Brazil. Braz J Infect Dis 9:341–347. https://doi.org/10.1590/s1413-86702005000400012
Nisalak A, Halstead SB, Singharaj P, Udomsakdi S, Nye SW, Vinijchaikul K (1970) Observations related to pathogenesis of dengue hemorrhagic fever. 3. Virologic studies of fatal disease. Yale J Biol Med 42:293–310
Limonta D, Capo V, Torres G, Perez AB, Guzman MG (2007) Apoptosis in tissues from fatal dengue shock syndrome. J Clin Virol 40:50–54. https://doi.org/10.1016/j.jcv.2007.04.024
Killen H, O’Sullivan MA (1993) Detection of dengue virus by in situ hybridization. J Virol Methods 41:135–146. https://doi.org/10.1016/0166-0934(93)90121-7
Rosen L, Drouet MT, Deubel V (1999) Detection of dengue virus RNA by reverse transcription-polymerase chain reaction in the liver and lymphoid organs but not in the brain in fatal human infection. Am J Trop Med Hyg 61:720–724. https://doi.org/10.4269/ajtmh.1999.61.720
Mongkolsapaya J, Duangchinda T, Dejnirattisai W, Vasanawathana S, Avirutnan P, Jairungsri A, Khemnu N, Tangthawornchaikul N, Chotiyarnwong P, Sae-Jang K, Koch M, Jones Y, McMichael A, Xu X, Malasit P, Screaton G (2006) T cell responses in dengue hemorrhagic fever: are cross-reactive T cells suboptimal? J Immunol 176:3821–3829. https://doi.org/10.4049/jimmunol.176.6.3821
Libraty DH, Young PR, Pickering D, Endy TP, Kalayanarooj S, Green S, Vaughn DW, Nisalak A, Ennis FA, Rothman AL (2002) High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J Infect Dis 186:1165–1168. https://doi.org/10.1086/343813
Paes MV, Pinhao AT, Barreto DF, Costa SM, Oliveira MP, Nogueira AC, Takiya CM, Farias-Filho JC, Schatzmayr HG, Alves AM, Barth OM (2005) Liver injury and viremia in mice infected with dengue-2 virus. Virology 338:236–246. https://doi.org/10.1016/j.virol.2005.04.042
Seneviratne SL, Malavige GN, de Silva HJ (2006) Pathogenesis of liver involvement during dengue viral infections. Trans R Soc Trop Med Hyg 100:608–614. https://doi.org/10.1016/j.trstmh.2005.10.007
Wichmann O, Gascon J, Schunk M, Puente S, Siikamaki H, Gjorup I, Lopez-Velez R, Clerinx J, Peyerl-Hoffmann G, Sundoy A, Genton B, Kern P, Calleri G, de Gorgolas M, Muhlberger N, Jelinek T, the European Network on Surveillance of Imported Infectious Diseases (2007) Severe dengue virus infection in travelers: risk factors and laboratory indicators. J Infect Dis 195:1089–1096. https://doi.org/10.1086/512680
Kyle JL, Beatty PR, Harris E (2007) Dengue virus infects macrophages and dendritic cells in a mouse model of infection. J Infect Dis 195:1808–1817. https://doi.org/10.1086/518007
de Souza LJ, Nogueira RM, Soares LC, Soares CE, Ribas BF, Alves FP, Vieira FR, Pessanha FE (2007) The impact of dengue on liver function as evaluated by aminotransferase levels. Braz J Infect Dis 11:407–410. https://doi.org/10.1590/s1413-86702007000400007
Ding X, Xu F, Chen H, Tesh RB, Xiao SY (2005) Apoptosis of hepatocytes caused by Punta Toro virus (Bunyaviridae: Phlebovirus) and its implication for Phlebovirus pathogenesis. Am J Pathol 167:1043–1049. https://doi.org/10.1016/S0002-9440(10)61193-5
Quaresma JA, Barros VL, Pagliari C, Fernandes ER, Andrade HF, Vasconcelos PF Jr, Duarte MI (2007) Hepatocyte lesions and cellular immune response in yellow fever infection. Trans R Soc Trop Med Hyg 101:161–168. https://doi.org/10.1016/j.trstmh.2006.02.019
Quaresma JA, Barros VL, Pagliari C, Fernandes ER, Guedes F, Takakura CF, Andrade HF, Vasconcelos PF Jr, Duarte MI (2006) Revisiting the liver in human yellow fever: virus-induced apoptosis in hepatocytes associated with TGF-beta, TNF-alpha and NK cells activity. Virology 345:22–30. https://doi.org/10.1016/j.virol.2005.09.058
Zampieri CA, Sullivan NJ, Nabel GJ (2007) Immunopathology of highly virulent pathogens: insights from Ebola virus. Nat Immunol 8:1159–1164. https://doi.org/10.1038/ni1519
Chen JP, Cosgriff TM (2000) Hemorrhagic fever virus-induced changes in hemostasis and vascular biology. Blood Coagul Fibrinolysis 11:461–483. https://doi.org/10.1097/00001721-200007000-00010
Bonner SM, O’Sullivan MA (1998) Endothelial cell monolayers as a model system to investigate dengue shock syndrome. J Virol Methods 71:159–167. https://doi.org/10.1016/s0166-0934(97)00211-5
Avirutnan P, Malasit P, Seliger B, Bhakdi S, Husmann M (1998) Dengue virus infection of human endothelial cells leads to chemokine production, complement activation, and apoptosis. J Immunol 161:6338–6346
Huang YH, Lei HY, Liu HS, Lin YS, Liu CC, Yeh TM (2000) Dengue virus infects human endothelial cells and induces IL-6 and IL-8 production. Am J Trop Med Hyg 63:71–75. https://doi.org/10.4269/ajtmh.2000.63.71
Sahaphong S, Riengrojpitak S, Bhamarapravati N, Chirachariyavej T (1980) Electron microscopic study of the vascular endothelial cell in dengue hemorrhagic fever. Southeast Asian J Trop Med Public Health 11:194–204
Boonpucknavig S, Boonpucknavig V, Bhamarapravati N, Nimmannitya S (1979) Immunofluorescence study of skin rash in patients with dengue hemorrhagic fever. Arch Pathol Lab Med 103:463–466
Rosenberg RD (2001) Vascular-bed-specific hemostasis and hypercoagulable states: clinical utility of activation peptide assays in predicting thrombotic events in different clinical populations. Thromb Haemost 86:41–50
Rosenberg RD, Aird WC (1999) Vascular-bed-specific hemostasis and hypercoagulable states. N Engl J Med 340:1555–1564. https://doi.org/10.1056/NEJM199905203402007
Chen HC, Hofman FM, Kung JT, Lin YD, Wu-Hsieh BA (2007) Both virus and tumor necrosis factor alpha are critical for endothelium damage in a mouse model of dengue virus-induced hemorrhage. J Virol 81:5518–5526. https://doi.org/10.1128/JVI.02575-06
Bethell DB, Gamble J, Pham PL, Nguyen MD, Tran TH, Ha TH, Tran TN, Dong TH, Gartside IB, White NJ, Day NP (2001) Noninvasive measurement of microvascular leakage in patients with dengue hemorrhagic fever. Clin Infect Dis 32:243–253. https://doi.org/10.1086/318453
Butthep P, Chunhakan S, Tangnararatchakit K, Yoksan S, Pattanapanyasat K, Chuansumrit A (2006) Elevated soluble thrombomodulin in the febrile stage related to patients at risk for dengue shock syndrome. Pediatr Infect Dis J 25:894–897. https://doi.org/10.1097/01.inf.0000237918.85330.b9
Murgue B, Cassar O, Deparis X (2001) Plasma concentrations of sVCAM-1 and severity of dengue infections. J Med Virol 65:97–104
Churdboonchart V, Bhamarapravati N, Futrakul P (1983) Crossed immunoelectrophoresis for the detection of split products of the third complement in dengue hemorrhagic fever. I. Observations in patients’ plasma. Am J Trop Med Hyg 32:569–576. https://doi.org/10.4269/ajtmh.1983.32.569
Nishioka K (1974) Serum complement level in dengue hemorrhagic fever. Allerg Immunol (Leipz) 21:385–392
Shaio MF, Chang FY, Hou SC (1992) Complement pathway activity in serum from patients with classical dengue fever. Trans R Soc Trop Med Hyg 86:672–675. https://doi.org/10.1016/0035-9203(92)90186-g
Markiewski MM, Nilsson B, Ekdahl KN, Mollnes TE, Lambris JD (2007) Complement and coagulation: strangers or partners in crime? Trends Immunol 28:184–192. https://doi.org/10.1016/j.it.2007.02.006
Avirutnan P, Punyadee N, Noisakran S, Komoltri C, Thiemmeca S, Auethavornanan K, Jairungsri A, Kanlaya R, Tangthawornchaikul N, Puttikhunt C, Pattanakitsakul SN, Yenchitsomanus PT, Mongkolsapaya J, Kasinrerk W, Sittisombut N, Husmann M, Blettner M, Vasanawathana S, Bhakdi S, Malasit P (2006) Vascular leakage in severe dengue virus infections: a potential role for the nonstructural viral protein NS1 and complement. J Infect Dis 193:1078–1088. https://doi.org/10.1086/500949
Feng JQ, Mozdzanowska K, Gerhard W (2002) Complement component C1q enhances the biological activity of influenza virus hemagglutinin-specific antibodies depending on their fine antigen specificity and heavy-chain isotype. J Virol 76:1369–1378. https://doi.org/10.1128/jvi.76.3.1369-1378.2002
Mehlhop E, Ansarah-Sobrinho C, Johnson S, Engle M, Fremont DH, Pierson TC, Diamond MS (2007) Complement protein C1q inhibits antibody-dependent enhancement of flavivirus infection in an IgG subclass specific manner. Cell Host Microbes 2:417–426. https://doi.org/10.1016/j.chom.2007.09.015
Koraka P, Suharti C, Setiati TE, Mairuhu AT, Van Gorp E, Hack CE, Juffrie M, Sutaryo J, Van Der Meer GM, Groen J, Osterhaus AD (2001) Kinetics of dengue virus-specific serum immunoglobulin classes and subclasses correlate with clinical outcome of infection. J Clin Microbiol 39:4332–4338. https://doi.org/10.1128/JCM.39.12.4332-4338.2001
Thein S, Aaskov J, Myint TT, Shwe TN, Saw TT, Zaw A (1993) Changes in levels of anti-dengue virus IgG subclasses in patients with disease of varying severity. J Med Virol 40:102–106. https://doi.org/10.1002/jmv.1890400205
Basu A, Chaturvedi UC (2008) Vascular endothelium: the battlefield of dengue viruses. FEMS Immunol Med Microbiol 53:287–299. https://doi.org/10.1111/j.1574-695X.2008.00420.x
Bozza FA, Cruz OG, Zagne SM, Azeredo EL, Nogueira RM, Assis EF, Bozza PT, Kubelka CF (2008) Multiplex cytokine profile from dengue patients: MIP-1beta and IFN-gamma as predictive factors for severity. BMC Infect Dis 8:86. https://doi.org/10.1186/1471-2334-8-86
Azeredo EL, Zagne SM, Santiago MA, Gouvea AS, Santana AA, Neves-Souza PC, Nogueira RM, Miagostovich MP, Kubelka CF (2001) Characterisation of lymphocyte response and cytokine patterns in patients with dengue fever. Immunobiology 204:494–507. https://doi.org/10.1078/0171-2985-00058
Chakravarti A, Kumaria R (2006) Circulating levels of tumour necrosis factor-alpha & interferon-gamma in patients with dengue & dengue haemorrhagic fever during an outbreak. Indian J Med Res 123:25–30
Juffrie M, Meer GM, Hack CE, Haasnoot K, Sutaryo A, Veerman AJ, Thijs LG (2001) Inflammatory mediators in dengue virus infection in children: interleukin-6 and its relation to C-reactive protein and secretory phospholipase A2. Am J Trop Med Hyg 65:70–75. https://doi.org/10.4269/ajtmh.2001.65.70
Laur F, Murgue B, Deparis X, Roche C, Cassar O, Chungue E (1998) Plasma levels of tumour necrosis factor alpha and transforming growth factor beta-1 in children with dengue 2 virus infection in French Polynesia. Trans R Soc Trop Med Hyg 92:654–656. https://doi.org/10.1016/s0035-9203(98)90800-8
Mustafa AS, Elbishbishi EA, Agarwal R, Chaturvedi UC (2001) Elevated levels of interleukin-13 and IL-18 in patients with dengue hemorrhagic fever. FEMS Immunol Med Microbiol 30:229–233. https://doi.org/10.1111/j.1574-695X.2001.tb01575.x
Nguyen TH, Lei HY, Nguyen TL, Lin YS, Huang KJ, Le BL, Lin CF, Yeh TM, Do QH, Vu TQ, Chen LC, Huang JH, Lam TM, Liu CC, Halstead SB (2004) Dengue hemorrhagic fever in infants:a study of clinical and cytokine profiles. J Infect Dis 189:221–232. https://doi.org/10.1086/380762
Lin CF, Chiu SC, Hsiao YL, Wan SW, Lei HY, Shiau AL, Liu HS, Yeh TM, Chen SH, Liu CC, Lin YS (2005) Expression of cytokine, chemokine, and adhesion molecules during endothelial cell activation induced by antibodies against dengue virus nonstructural protein 1. J Immunol 174:395–403. https://doi.org/10.4049/jimmunol.174.1.395
Boonpucknavig S, Boonpucknavig V, Bhamarapravati N, Nimmannitya S (1979) Immunofluorescence study of skin rash in patients with dengue hemorrhagic fever. Arch Pathol Lab Med 103:463–466
Azizan A, Sweat J, Espino C, Gemmer J, Stark L, Kazanis D (2006) Differential proinflammatory and angiogenesis-specific cytokine production in human pulmonary endothelial cells, HPMEC-ST1.6R infected with dengue-2 and dengue-3 virus. J Virol Methods 138:211–217. https://doi.org/10.1016/j.jviromet.2006.08.010
Fernandez-Mestre MT, Gendzekhadze K, Rivas-Vetencourt P, Layrisse Z (2004) TNF-alpha-308A allele, a possible severity risk factor of hemorrhagic manifestation in dengue fever patients. Tissue Antigens 64:469–472. https://doi.org/10.1111/j.1399-0039.2004.00304.x
Dewi BE, Takasaki T, Kurane I (2004) In vitro assessment of human endothelial cell permeability: effects of inflammatory cytokines and dengue virus infection. J Virol Methods 121:171–180. https://doi.org/10.1016/j.jviromet.2004.06.013
Shen BQ, Lee DY, Cortopassi KM, Damico LA, Zioncheck TF (2001) Vascular endothelial growth factor KDR receptor signaling potentiates tumor necrosis factor-induced tissue factor expression in endothelial cells. J Biol Chem 276:5281–5286. https://doi.org/10.1074/jbc.M007969200
Gomez A, Serrano A, Salero E, Tovar A, Amescua G, Galor A, Keane RW, de Rivero Vaccari JP, Sabater AL (2021) Tumor necrosis factor-alpha and interferon-gamma induce inflammasome-mediated corneal endothelial cell death. Exp Eye Res 207:108574. https://doi.org/10.1016/j.exer.2021.108574
Stamatovic SM, Keep RF, Kunkel SL, Andjelkovic AV (2003) Potential role of MCP-1 in endothelial cell tight junction ‘opening’: signaling via Rho and Rho kinase. J Cell Sci 116:4615–4628. https://doi.org/10.1242/jcs.00755
Luplertlop N, Misse D, Bray D, Deleuze V, Gonzalez JP, Leardkamolkarn V, Yssel H, Veas F (2006) Dengue-virus-infected dendritic cells trigger vascular leakage through metalloproteinase overproduction. EMBO Rep 7:1176–1181. https://doi.org/10.1038/sj.embor.7400814
Agarwal R, Chaturvedi UC, Misra A, Mukerjee R, Kapoor S, Nagar R, Tandon R, Mathur A (1998) Production of cytotoxic factor by peripheral blood mononuclear cells (PBMC) in patients with dengue haemorrhagic fever. Clin Exp Immunol 112:477–481. https://doi.org/10.1046/j.1365-2249.1998.00598.x
Chaturvedi UC, Elbishbishi EA, Agarwal R, Mustafa AS (2001) Cytotoxic factor-autoantibodies: possible role in the pathogenesis of dengue haemorrhagic fever. FEMS Immunol Med Microbiol 30:181–186. https://doi.org/10.1111/j.1574-695X.2001.tb01568.x
Mongkolsapaya J, Duangchinda T, Dejnirattisai W, Vasanawathana S, Avirutnan P, Jairungsri A, Khemnu N, Tangthawornchaikul N, Chotiyarnwong P, Sae-Jang K, Koch M, Jones Y, McMichael A, Xu X, Malasit P, Screaton G (2006) T cell responses in dengue hemorrhagic fever: are cross-reactive T cells suboptimal? J Immunol 176:3821–3829. https://doi.org/10.4049/jimmunol.176.6.3821
An J, Zhou DS, Zhang JL, Morida H, Wang JL, Yasui K (2004) Dengue-specific CD8_ T cells have both protective and pathogenic roles in dengue virus infection. Immunol Lett 95:167–174. https://doi.org/10.1016/j.imlet.2004.07.006
Snyder JT, Alexander-Miller MA, Berzofskyl JA, Belyakov IM (2003) Molecular mechanisms and biological significance of CTL avidity. Curr HIV Res 1:287–294. https://doi.org/10.2174/1570162033485230
Klenerman P, Zinkernagel RM (1998) Original antigenic sin impairs cytotoxic T lymphocyte responses to viruses bearing variant epitopes. Nature 394:482–485. https://doi.org/10.1038/28860
Mangada MM, Endy TP, Nisalak A, Chunsuttiwat S, Vaughn DW, Libraty DH, Green S, Ennis FA, Rothman AL (2002) Denguespecific T cell responses in peripheral blood mononuclear cells obtained prior to secondary dengue virus infections in Thai schoolchildren. J Infect Dis 185:1697–1703. https://doi.org/10.1086/340822
Narayan R, Tripathi S (2020) Intrinsic ADE: The Dark Side of Antibody Dependent Enhancement During Dengue Infection. Front Cell Infect Microbiol 10:580096. https://doi.org/10.3389/fcimb.2020.580096
Lidbury BA, Mahalingam S (2000) Specific ablation of antiviral gene expression in macrophages by antibody-dependent enhancement of Ross River virus infection. J Virol 74:8376–8381. https://doi.org/10.1128/jvi.74.18.8376-8381.2000
Mahalingam S, Lidbury BA (2002) Suppression of lipopolysaccharide- induced antiviral transcription factor (STAT-1 and NF-kappa B) complexes y antibody-dependent enhancement of macrophage infection by Ross River virus. Proc Natl Acad Sci USA 99:13819–13824. https://doi.org/10.1073/pnas.202415999
Chareonsirisuthigul T, Kalayanarooj S, Ubol S (2007) Dengue virus (DENV) antibody-dependent enhancement of infection upregulates the production of anti-inflammatory cytokines, but suppresses anti-DENV free radical and pro-inflammatory cytokine production, in THP-1 cells. J Gen Virol 88:365–375. https://doi.org/10.1099/vir.0.82537-0
Thomas L, Verlaeten O, Cabie A, Kaidomar S, Moravie V, Martial J, Najioullah F, Plumelle Y, Fonteau C, Dussart P, Cesaire R (2008) Influence of the dengue serotype, previous dengue infection, and plasma viral load on clinical presentation and outcome during a dengue-2 and dengue-4 co-epidemic. Am J Trop Med Hyg 78:990–998
Libraty DH, Endy TP, Houng HS, Green S, Kalayanarooj S, Suntayakorn S, Chansiriwongs W, Vaughn DW, Nisalak A, Ennis FA, Rothman AL (2002b) Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. J Infect Dis 185:1213–1221. https://doi.org/10.1086/340365
Simmons CP, Chau TN, Thuy TT, Tuan NM, Hoang DM, Thien NT, Lien le B, Quy NT, Hieu NT, Hien TT, McElnea C, Young P, Whitehead S, Hung NT, Farrar J (2007) Maternal antibody and viral factors in the pathogenesis of dengue virus in infants. J Infect Dis 196:416–424. https://doi.org/10.1086/519170
Chungue E, Poli L, Roche C, Gestas P, Glaziou P, Markoff LJ (1994) Correlation between detection of plasminogen cross-reactive antibodies and hemorrhage in dengue virus infection. J Infect Dis 170:1304–1307. https://doi.org/10.1093/infdis/170.5.1304
Falconar AK (1997) The dengue virus nonstructural-1 protein (NS1) generates antibodies to common epitopes on human blood clotting, integrin/ adhesin proteins and binds to human endothelial cells: potential implications in haemorrhagic fever pathogenesis. Arch Virol 142:897–916. https://doi.org/10.1007/s007050050127
Huang YH, Chang BI, Lei HY, Liu HS, Liu CC, Wu HL, Yeh M (1997) Antibodies against dengue virus E protein peptide bind to human plasminogen and inhibit plasmin activity. Clin Exp Immunol 110:35–40. https://doi.org/10.1046/j.1365-2249.1997.4991398.x
Markoff LJ, Innis BL, Houghten R, Henchal LS (1991) Development of cross-reactive antibodies to plasminogen during the immune response to dengue virus infection. J Infect Dis 164:294–301. https://doi.org/10.1093/infdis/164.2.294
Lin CF, Lei HY, Shiau AL, Liu CC, Liu HS, Yeh TM, Chen SH, Lin YS (2003) Antibodies from dengue patient sera cross-react with endothelial cells and induce damage. J Med Virol 69:82–90. https://doi.org/10.1002/jmv.10261
Nachman RL, Rafii S (2008) Platelets, petechiae, and preservation of the vascular wall. N Engl J Med 359:1261–1270. https://doi.org/10.1056/NEJMra0800887
Oishi K, Inoue S, Cinco MT, Dimaano EM, Alera MT, Alfon JA, Abanes F, Cruz DJ, Matias RR, Matsuura H, Hasebe F, Tanimura S, Kumatori A, Morita K, Natividad FF, Nagatake T (2003) Correlation between increased platelet-associated IgG and thrombocytopenia in secondary dengue virus infections. J Med Virol 71:259–264. https://doi.org/10.1002/jmv.10478
Sun DS, King CC, Huang HS, Shih YL, Lee CC, Tsai WJ, Yu CC, Chang HH (2007) Antiplatelet autoantibodies elicited by dengue virus non-structural protein 1 cause thrombocytopenia and mortality in mice. J Thromb Haemost 5:2291–2299. https://doi.org/10.1111/j.1538-7836.2007.02754.x
Shu PY, Chen LK, Chang SF, Yueh YY, Chow L, Chien LJ, Chin C, Lin TH, Huang JH (2000) Dengue NS1-specific antibody responses: isotype distribution and serotyping in patients with dengue fever and dengue hemorrhagic fever. J Med Virol 62:224–232. https://doi.org/10.1002/1096-9071(200010)62:2<224::aid-jmv14>3.0.co;2-c
Ubol S, Chareonsirisuthigul T, Kasisith J, Klungthong C (2008) Clinical isolates of dengue virus with distinctive susceptibility to nitric oxide radical induce differential gene responses in THP-1 cells. Virology 376:290–296. https://doi.org/10.1016/j.virol.2008.03.030
Sanchez IJ, Ruiz BH (1996) A single nucleotide change in the E protein gene of dengue virus 2 Mexican strain affects neurovirulence in mice. J Gen Virol 77:2541–2545. https://doi.org/10.1099/0022-1317-77-10-2541
Leitmeyer KC, Vaughn DW, Watts DM, Salas R, Villalobos I, Ramos CC, Rico-Hesse R (1999) Dengue virus structural differences that correlate with pathogenesis. J Virol 73:4738–4747. https://doi.org/10.1128/JVI.73.6.4738-4747.1999
Cologna R, Rico-Hesse R (2003) American genotype structures decrease dengue virus output from human monocytes and dendritic cells. J Virol 77:3929–3938. https://doi.org/10.1128/jvi.77.7.3929-3938.2003
Kouri GP, Guzman MG, Bravo JR (1987) Why dengue haemorrhagic fever in Cuba? 2. An integral analysis. Trans R Soc Trop Med Hyg 81:821–823. https://doi.org/10.1016/0035-9203(87)90042-3
Kouri GP, Guzman MG, Bravo JR, Triana C (1989) Dengue haemorrhagic fever/dengue shock syndrome: lessons from the Cuban epidemic, 1981. Bull WH O 67:375–380
Ong A, Sandar M, Chen MI, Sin LY (2007) Fatal dengue hemorrhagic fever in adults during a dengue epidemic in Singapore. Int J Infect Dis 11:263–267. https://doi.org/10.1016/j.ijid.2006.02.012
Halstead SB Dengue. Lancet. 370:1644–1652., Guzman MG, Kouri G (2007) 2003. Dengue and dengue hemorrhagic fever in the Americas: lessons and challenges. J Clin Virol 27:1–13. https://doi.org/10.1016/s1386-6532(03)00010-6
Guzman MG, Kouri G, Bravo J, Valdes L, Vazquez S, Halstead SB (2002) Effect of age on outcome of secondary dengue 2 infections. Int J Infect Dis 6:118–124. https://doi.org/10.1016/s1201-9712(02)90072-x
LaFleur C, Granados J, Vargas-Alarcon G, Ruiz-Morales J, Villarreal- Garza C, Higuera L, Hernandez-Pacheco G, Cutino-Moguel T, Rangel H, Figueroa R, Acosta M, Lazcano E, Ramos C (2002) HLA-DR antigen frequencies in Mexican patients with dengue virus infection: HLADR4 as a possible genetic resistance factor for dengue hemorrhagic fever. Hum Immunol 63:1039–1044. https://doi.org/10.1016/s0198-8859(02)00682-1
Loke H, Bethell DB, Phuong CX, Dung M, Schneider J, White NJ, Day NP, Farrar J, Hill AV (2001) Strong HLA class I-restricted T cell responses in dengue hemorrhagic fever: a double-edged sword? J Infect Dis 184:1369–1373. https://doi.org/10.1086/324320
Polizel JR, Bueno D, Visentainer JE, Sell AM, Borelli SD, Tsuneto LT, Dalalio MM, Coimbra MT, Moliterno RA (2004) Association of human leukocyte antigen DQ1 and dengue fever in a white Southern Brazilian population. Mem Inst Oswaldo Cruz 99:559–562. https://doi.org/10.1590/s0074-02762004000600003
Stephens HA, Klaythong R, Sirikong M, Vaughn DW, Green S, Kalayanarooj S, Endy TP, Libraty DH, Nisalak A, Innis BL, Rothman AL, Ennis FA, Chandanayingyong D (2002) HLA-A and -B allele associations with secondary dengue virus infections correlate with disease severity and the infecting viral serotype in ethnic Thais. Tissue Antigens 60:309–318. https://doi.org/10.1034/j.1399-0039.2002.600405.x
Zivna I, Green S, Vaughn DW, Kalayanarooj S, Stephens HA, Chandanayingyong D, Nisalak A, Ennis FA, Rothman AL (2002) T cell responses to an HLA-B*07-restricted epitope on the dengue NS3 protein correlate with disease severity. J Immunol 168:5959–5965. https://doi.org/10.4049/jimmunol.168.11.5959
Loke H, Bethell D, Phuong CX, Day N, White N, Farrar J, Hill A (2002) Susceptibility to dengue hemorrhagic fever in Vietnam: evidence of an association with variation in the vitamin d receptor and Fc gamma receptor IIa genes. Am J Trop Med Hyg 67:102–106. https://doi.org/10.4269/ajtmh.2002.67.102
Chen RF, Wang L, Cheng JT, Chuang H, Chang JC, Liu JW, Lin IC, Yang KD (2009) Combination of CTLA-4 and TGFbeta1 gene polymorphisms associated with dengue hemorrhagic fever and virus load in a dengue-2 outbreak. Clin Immunol 131:404–409. https://doi.org/10.1016/j.clim.2009.01.015
Danilczyk U, Penninger JM (2006) Angiotensin-converting enzyme II in the heart and the kidney. Circ Res 98:463–471. https://doi.org/10.1161/01.RES.0000205761.22353.5f
Huang XR, Chen WY, Truong LD, Lan HY (2003) Chymase is upregulated in diabetic nephropathy: implications for an alternative pathway of angiotensin II-mediated diabetic renal and vascular disease. J Am Soc Nephrol 14:1738–1747. https://doi.org/10.1097/01.asn.0000071512.93927.4e
Bacani C, Frishman WH (2006) Chymase: a new pharmacologic target in cardiovascular disease. Cardiol Rev 14:187–193. https://doi.org/10.1097/01.crd.0000195220.62533.c5
Cesari M, Rossi GP, Pessina AC (2002) Biological properties of the angiotensin peptides other than angiotensin II: implications for hypertension and cardiovascular diseases. J Hypertens 20:793–799. https://doi.org/10.1097/00004872-200205000-00002
Hamilton TA, Handa RK, Harding JW, Wright JW (2001) A role for angiotensin IV/AT4 system in mediating natiuresis in the rat. Peptides 22:935–944. https://doi.org/10.1016/s0196-9781(01)00405-3
Kramar EA, Harding JW, Wright JW (1997) Angiotensin II- and IV-induced changes in cerebral blood flow. Roles of AT1 and AT2, and AT4 receptor subtypes. Regul Pept 68:131–138. https://doi.org/10.1016/s0167-0115(96)02116-7
Van Kats JP, Danser AH, van Meegen JR, Sassen LM, Verdouw PD, Schalekamp MA (1998) Angiotensin production by the heart: a quantitative study in pigs with the use of radiolabeled angiotensin infusion. Circulation 98:73–81. https://doi.org/10.1161/01.cir.98.1.73
Kobori H, Pieto-Carrasquero MC, Ozawa Y, Navar LG (2004) AT1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin II dependent hypertension. Hypertension 43:1126–1132. https://doi.org/10.1161/01.HYP.0000122875.91100.28
Moulik S, Speth RC, Turner BB, Rowe BP (2002) Angiotensin II receptor subtype distribution in the rabbit brain. Exp Brain Res 142:275–283. https://doi.org/10.1007/s00221-001-0940-5
Ghiani BU, Masini MA (1995) Angiotensin II bindings sites in the rat pancreas and their modulation after sodium loading and depletion. Comp Biochem Physiol A Physiol 111:439–444. https://doi.org/10.1016/0300-9629(95)00030-b
Karlsson C, Lindell K, Ottosson M, Sjostrom L, Carlsson B, Carlsso L (1998) Human Adipose Tissue Expresses Angiotensinogen and Enzymes Required for Its Conversion to Angiotensin II. J Clin Endocrinol Metabol 83:3925–3929. https://doi.org/10.1210/jcem.83.11.5276
de Mello W (2003) Effect of extracellular and intracellular angiotensin on heart cell function; on the cardiac renin-angiotensin system. Regul Pept 114:87–90. https://doi.org/10.1016/s0167-0115(03)00121-6
Re RN, Cook JL (2006) The intracrine hypothesis: an update. Regul Pept 133:1–9. https://doi.org/10.1016/j.regpep.2005.09.012
Rüster C, Wolf G (2013) The role of the renin-angiotensin-aldosterone system in obesity-related renal diseases. Semin Nephrol 33:44–53. https://doi.org/10.1016/j.semnephrol.2012.12.002
Ruster C, Wolf G (2006) Renin-angiotensin-aldosterone system and progression of renal disease. J Am Soc Nephrol 17:2985–2991. https://doi.org/10.1681/ASN.2006040356
Hunyady L, Catt KJ (2006) Pleiotropic AT1 receptor signaling pathways mediating 10.1016/j.semnephrol.2012.12.002.physiological and pathogenic actions of angiotensin II. Mol Endocrinol 20:953–970. https://doi.org/10.1210/me.2004-0536
Porrello ER, Delbridge LM, Thomas WG (2009) The angiotensin II type 2 (AT2) receptor: an enigmatic seven transmembrane receptor. Front BioSci 14:958–972. https://doi.org/10.2741/3289
Ito N, Ohishi M, Yamamoto K, Tatara Y, Shiota A, Hayashi N, Komai N, Yanagitani Y, Rakugi H, Ogihara T (2007) Renin-angiotensin inhibition reverses advanced cardiac remodeling in aging spontaneously hypertensive rats. Am J Hypertens 20:792–799. https://doi.org/10.1016/j.amjhyper.2007.02.004
Thekkumkara TJ, Cookson R, Linas SL (1998) Angiotensin (AT1A) receptor mediated increases in transcellular sodium transport in proximal tubule cells. Am J Physiol 274:F897–F905. https://doi.org/10.1152/ajprenal.1998.274.5.F897
Aguilera G (1992) Role of angiotensin II receptor subtypes on the regulation of aldosterone secretion in the adrenal glomerulosa zone in the rat. Mol Cell Endocrinol 90:53–60. https://doi.org/10.1016/0303-7207(92)90101-b
Davisson RL, Oliverio MI, Coffman TM, Sigmund CD (2000) Divergent functions of angiotensin II receptor isoforms in the brain. J Clin Invest 106:103–106. https://doi.org/10.1172/JCI10022
Oliverio MI, Coffman TM (2000) Angiotensin II receptor physiology using gene targeting. News Physiol Sci 15:171–175. https://doi.org/10.1152/physiologyonline.2000.15.4.171
Schulman IH, Raij L (2008) The angiotensin II type 2 receptor: what is its clinical significance? Curr Hypertens Rep 10:188–193. https://doi.org/10.1007/s11906-008-0036-8
Esteban V, Lorenzo O, Ruperez M, Suzuki Y, Mezzano S, Blanco J, Kretzler M, Sugaya T, Egido J, Ruiz-Ortega M (2004) Angiotensin II, via AT1 and AT2 receptors and NF-kB pathway, regulates the inflammatory response in unilateral ureteral obstruction. J Am Soc Nephrol 15:1514–1529. https://doi.org/10.1097/01.asn.0000130564.75008.f5
Ruiz-Ortega M, Esteban V, Suzuki Y, Ruperez M, Mezzano S, Ardiles L, Justo P, Ortiz A, Egido J (2003) Renal expression of angiotensin type 2 (AT2) receptors during kidney damage. Kidney Int Suppl 86:S21–S26. https://doi.org/10.1046/j.1523-1755.64.s86.5.x
de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T (2000) International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev 52:415–472
Marchesi C, Paradis P, Schiffrin EL (2008) Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol Sci 29:367–374. https://doi.org/10.1016/j.tips.2008.05.003
Chua CC, Hamdy RC, Chua BH (1998) Upregulation of vascular endothelial growth factor by angiotensin II in rat heart endothelial cells. Biochim Biophys Acta 1401:187–194. https://doi.org/10.1016/s0167-4889(97)00129-8
Kitayama H, Maeshima Y, Takazawa Y, Yamamoto Y, Wu Y, Ichinose K, Hirokoshi K, Sugiyama H, Yamasaki Y, Makino H (2006) Regulation of angiogenic factors in angiotensin II infusion model in association with tubulointerstitial injuries. Am J Hypertens 19:718–727. https://doi.org/10.1016/j.amjhyper.2005.09.022
Suzuki Y, Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Egido J (2003) Inflammation and angiotensin II. Int J Biochem Cell Biol 35:881–900. https://doi.org/10.1016/s1357-2725(02)00271-6
Alvarez A, Cerda´-Nicola´s M, Abu N, Nabah Y, Mata M, Issekutz AC, Panés J, Lobb RR, Sanz MJ (2004) Direct evidence of leukocyte adhesion in arterioles by angiotensin II. Blood 104:402–408. https://doi.org/10.1182/blood-2003-08-2974
Piqueras L, Kubes P, Alvarez A, O’Connor E, Issekutz AC, Esplugues JV, Sanz MJ (2000) Angiotensin II induces leukocyte-endothelial cell interactions in vivo via AT(1) and AT(2) receptor-mediated P-selectin upregulation. Circulation 102:2118–2123. https://doi.org/10.1161/01.cir.102.17.2118
Pueyo ME, Gonzalez W, Nicoletti A, Savoie F, Arnal JF, Michel JB (2000) Angiotensin stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-kappaB activation induced by intracellular oxidative stress. Artherocler Thromb Vasc Biol 20:645–651. https://doi.org/10.1161/01.atv.20.3.645
Crowley SD, Frey CW, Gould SK, Griffiths R, Ruiz P, Burchette JL, Howell DN, Makhanova N, Yan M, Kim HS, Tharaux PL, Coffman TM (2008) Stimulation of lymphocyte responses by angiotensin II promotes kidney injury in hypertension. Am J Physiol Renal Physiol 295:F515–F524. https://doi.org/10.1152/ajprenal.00527.2007
Jurewicz M, McDermott DH, Sechler JM, Tinckam K, Takakura A, Carpenter CB, Milford E, Abdi R (2007) Human T and natural killer cells possess a functional renin-angiotensin system: further mechanisms of angiotensin II induced inflammation. J Am Soc Nephrol 18:1093–10102. https://doi.org/10.1681/ASN.2006070707
Kvakan H, Kleinewietfeld M, Qadri F, Park JK, Fischer R, Schwarz I, Rahn HP, Plehm R, Wellner M, Elitok S, Gratze P, Luft R, Muller FC DN (2009) Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation 119:2904–2912. https://doi.org/10.1161/CIRCULATIONAHA.108.832782
Welch WJ (2008) Angiotensin II-dependent superoxide: effects on hypertension and vascular dysfunction. Hypertension 52:51–56. https://doi.org/10.1161/HYPERTENSIONAHA.107.090472
Wu R, Laplante MA, de Champlain J (2005) Cyclooxygenase-2 inhibitors attenuate angiotensin II-induced oxidative stress, hypertension, and cardiac hypertrophy in rats. Hypertension 45:1139–1144. https://doi.org/10.1161/01.HYP.0000164572.92049.29
Wen Y, Liu Y, Tang T, Lv L, Liu H, Ma K, Liu B (2016) NLRP3 inflammasome activation is involved in Ang II-induced kidney damage via mitochondrial dysfunction. Oncotarget 7:54290–54302. https://doi.org/10.18632/oncotarget.11091
Thakur S, Li L, Gupta S (2014) NF-κB-mediated integrin-linked kinase regulation in angiotensin II-induced pro-fibrotic process in cardiac fibroblasts. Life Sci 107:68–75. https://doi.org/10.1016/j.lfs.2014.04.030
Weber KT, Swamynathan SK, Guntaka RV, Sun Y (1999) Angiotensin II and Extracellular Matrix Homeostasis. J Biochem Cell Biol 31:395–403. https://doi.org/10.1016/s1357-2725(98)00125-3
Than A, Leow MK, Chen P (2013) Control of adipogenesis by the autocrine interplays between angiotensin 1–7/Mas receptor and angiotensin II/AT1 receptor signaling pathways. J Biol Chem 288:15520–15531. https://doi.org/10.1074/jbc.M113.459792
Sharma AM, Engeli S (2006) The role of renin-angiotensin system blockade in the management of hypertension associated with the cardiometabolic syndrome. J Cardiometab Syndr 1:29–35. https://doi.org/10.1111/j.0197-3118.2006.05422.x
Chalmers L, Kaskel FJ, Bamgbola O (2006) The role of obesity and its bioclinical correlates in the progression of chronic kidney disease. Adv Chronic Kidney Dis 13:352–364. https://doi.org/10.1053/j.ackd.2006.07.010
Gao N, Wang H, Zhang X, Yang Z (2015) The inhibitory effect of angiotensin II on BKCa channels in podocytes via oxidative stress. Mol Cell Biochem 398:217–222. https://doi.org/10.1007/s11010-014-2221-1
Saginova EA, Fedorova EIu, Fomin VV, Moiseev SV, Minakova EG, Gitel’ EP, Samokhodskaia LM, Kutyrina IM, Mukhin NA (2006) [Development of renal affection in obese patients]. Ter Arkh 78:36–41
Hongo M, Ishizaka N, Furuta K, Yahagi N, Saito K, Sakurai R, Matsuzaki G, Koike K, Nagai R (2009) Administration of angiotensin II, but not catecholamines, induces accumulation of lipids in the rat heart. Eur J Pharmacol 604:87–92. https://doi.org/10.1016/j.ejphar.2008.12.006
Mayor F Jr, Cruces-Sande M, Arcones AC, Vila-Bedmar R, Briones AM, Salaices M, Murga C (2018) G protein-coupled receptor kinase 2 (GRK2) as an integrative signalling node in the regulation of cardiovascular function and metabolic homeostasis. Cell Signal 41:25–32. https://doi.org/10.1016/j.cellsig.2017.04.002
Glenn DJ, Cardema MC, Ni W, Zhang Y, Yeghiazarians Y, Grapov D, Fiehn O, Gardner DG (2015) Cardiac steatosis potentiates angiotensin II effects in the heart. Am J Physiol Heart Circ Physiol 308:H339–350. https://doi.org/10.1152/ajpheart.00742.2014
Kintscher U, Lyon CJ, Law RE (2004) Angiotensin II, PPAR-gamma and atherosclerosis. Front Biosci 9:359–369. https://doi.org/10.2741/1225
Schuchard J, Winkler M, Stölting I, Schuster F, Vogt FM, Barkhausen J, Thorns C, Santos RA, Bader M, Raasch W (2015) Lack of weight gain after angiotensin AT1 receptor blockade in diet-induced obesity is partly mediated by an angiotensin-(1–7)/Mas-dependent pathway. Br J Pharmacol 172:3764–3778. https://doi.org/10.1111/bph.13172
Kochueva M, Sukhonos V, Shalimova A, Psareva V, Kirichenko N (2014) State of integral remodeling parameters of target organs in patients with essential hypertension and obesity. Georgian Med News 231:26–30
Frohlich ED (2002) Clinical management of the obese hypertensive patient. Cardiol Rev 10:127–138. https://doi.org/10.1097/00045415-200205000-00001
Xue B, Yu Y, Zhang Z, Guo F, Beltz TG, Thunhorst RL, Felder RB, Johnson AK (2016b) Leptin Mediates High-Fat Diet Sensitization of Angiotensin II-Elicited Hypertension by Upregulating the Brain Renin-Angiotensin System and Inflammation. Hypertension 67:970–976. https://doi.org/10.1161/HYPERTENSIONAHA.115.06736
Deji N, Kume S, Araki S, Isshiki K, Araki H, Chin-Kanasaki M, Tanaka Y, Nishiyama A, Koya D, Haneda M, Kashiwagi A, Maegawa H, Uzu T (2012) Role of angiotensin II-mediated AMPK inactivation on obesity-related salt-sensitive hypertension. Biochem Biophys Res Commun 418:559–564. https://doi.org/10.1016/j.bbrc.2012.01.070
Vaidya A, Forman JP, Williams JS (2011) Vitamin D and the vascular sensitivity to angiotensin II in obese Caucasians with hypertension. J Hum Hypertens 25:672–678. https://doi.org/10.1038/jhh.2010.110
Mutch NJ, Wilson HM, Booth NA (2001) Plasminogen Activator inhibitor-1 and Haemostasis in Obesity. Proc Nutr Soc 6:341–347. https://doi.org/10.1079/pns200199
Skurk T, Lee YM, Hauner H (2001) Angiotensin II and its metabolites stimulate PAI-1 protein release from human adipocytes in primary culture. Hypertension 37:1336–1340. https://doi.org/10.1161/01.hyp.37.5.1336
Kuba K, Imai Y, Ohto-Nakanishi T, Penninger JM (2010) Trilogía de ACE2: una peptidasa en el sistema renina-angiotensina, un receptor de SARS y un compañero para los transportadores de aminoácidos. Pharmacol Ther 128:119–128. https://doi.org/10.1016/j.pharmthera.2010.06.003
Yang P, Gu H, Zhao Z, Wang W, Cao B, Lai C, Yang X, Zhang L, Duan Y, Zhang S, Chen W, Zhen W, Cai M, Penninger JM, Jiang C, Wang X (2014) La enzima convertidora de angiotensina 2 (ACE2) media la lesión pulmonar aguda inducida por el virus de la influenza H7N9. Sci Rep 4:7027–7032. https://doi.org/10.1038/srep07027
Moskowitz DW, Johnson FE (2004) The central role of angiotensin I-converting enzyme in vertebrate pathophysiology. Curr Top Med Chem 4(13):1433–1454. https://doi.org/10.2174/1568026043387818
Loe MWC, Hao E, Chen M, Cong Li RChingH, Lee IXinYu, Zhu ZY, Teo Wei-Xin Chin, Xiaotao Hou, JiaGang Deng, Justin Jang Hann Chu (2020) Betulinic acid exhibits antiviral effects against dengue virus infection. Antiviral Res 184:104954. https://doi.org/10.1016/j.antiviral.2020.104954
Halstead SB (1989) Antibody, macrophages, dengue virus infection, shock, and hemorrhage: a pathogenetic cascade. Rev Infect Dis 11 Suppl 4S830–839. https://doi.org/10.1093/clinids/11.supplement_4.s830
Mosquera JA, Hernandez JP, Valero N, Espina LM, Añez GJ (2005) Ultrastructural studies on dengue virus type 2 infection of cultured human monocytes. Virol J 2:26. https://doi.org/10.1186/1743-422X-2-26
Benigni A, Cassis P, Remuzzi G (2010) Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med 2:247–257. https://doi.org/10.1002/emmm.201000080
Ruiz-Ortega M, Esteban V, Suzuki Y, Ruperez M, Mezzano S, Ardiles L, Justo P, Ortiz A, Egidio J (2003) Renal expression of angiotensin type 2 (AT2) receptors during kidney damage. Kidney Int Suppl 86:S21–S26. https://doi.org/10.1046/j.1523-1755.64.s86.5.x
Duran A, Valero N, Mosquera J, Fuenmayor E, Alvarez-Mon M (2017) Gefitinib and pyrrolidine dithiocarbamate decrease viral replication and cytokine production in dengue virus infected human monocyte cultures. Life Sci 191:180–185. https://doi.org/10.1016/j.lfs.2017.10.027
Alanazi WA, Alhamami HN, Alharbi M, Alhazzani K, Alanazi AS, Alsanea S, Ali N, Alasmari AF, Alanazi AZ, Alotaibi MR, Saudi MA (2022) Angiotensin II type 1 receptor blockade attenuates gefitinib-induced cardiac hypertrophy via adjusting angiotensin II-mediated oxidative stress and JNK/P38 MAPK pathway in a rat model. Pharm J 30:1159–1169. https://doi.org/10.1016/j.jsps.2022.06.020
Vuong NL, Lam PK, Ming DKY, Le Huynh Thi HTL, Nguyet Minh Nguyen NM, Dong Thi Hoai Tam DTH, Hue DDT, Chau NW, Chanpheaktra N, Lum LCS, Pleite´s E, Simmons CP, Rosenberger KD, Thomas Jaenisch T, Bell D, Acestor N, Halleux C, Olliaro PL, Bridget A, Wills BA, Ronald B, Geskus RB, Yacoub S (2021) Combination of inflammatory and vascular markers in the febrile phase of dengue is associated with more severe outcomes. eLife 10:e67460. https://doi.org/10.7554/eLife.67460
Patro ARK, Mohanty S, Prusty BK, Singh DK, Gaikwad S, Saswat T (2019) Cytokine signature associated with disease severity in dengue. Viruses. [cited 2020 May 8];11. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6357178/
Valero N, Mosquera J, Torres M, Duran A, Velastegui M, Reyes J, Fernandez M, Fernandez G, Veliz T (2019) Increased serum ferritin and interleukin-18 levels in children with dengue. Braz J Microbiol 50:649–656. https://doi.org/10.1007/s42770-019-00105-2
Valero N, Mosquera J, Añez G, Levy A, Marcucci R, de Mon MA (2013) Differential oxidative stress induced by dengue virus in monocytes from human neonates, adult and elderly individuals. PLoS ONE 8:e73221. https://doi.org/10.1371/journal.pone.0073221
Arias J, Valero N, Mosquera J, Montiel M, Reyes E, Larreal Y, Alvarez-Mon M (2014) Increased expression of cytokines, soluble cytokine receptors, soluble apoptosis ligand and apoptosis in dengue. Virology 452–453:42–51. https://doi.org/10.1016/j.virol.2013.12.027
Duran A, Valero N, Mosquera J, Pons H, Torres M, Alcocer S, Castillo JL (2013) Dengue nonstructural protein-1 status is not associated to circulating levels of interleukin-17, C-reactive protein and complement in children with acute dengue. J Clin Virol 56:199–206. https://doi.org/10.1016/j.jcv.2012.11.007
Valero N, Mosquera J, Levy A, Añez G, Marcucci R, Alvarez-Mon M (2014) Differential induction of cytokines by human neonatal, adult, and elderly monocyte/macrophages infected with dengue virus. Viral Immunol 27:151–159. https://doi.org/10.1089/vim.2013.0123
Levy A, Valero N, Espina LM, Añez G, Arias J, Mosquera J (2010) Increment of interleukin 6, tumour necrosis factor alpha, nitric oxide, C-reactive protein and apoptosis in dengue. Trans R Soc Trop Med Hyg 104:16–23. https://doi.org/10.1016/j.trstmh.2009.06.013
Valero N, Larreal Y, Espina LM, Reyes I, Maldonado M, Mosquera J (2008) Elevated levels of interleukin-2 receptor and intercellular adhesion molecule 1 in sera from a venezuelan cohort of patients with dengue. Arch Virol 153:199–203. https://doi.org/10.1007/s00705-007-1080-4
Valero N, Espina LM, Añez G, Torres E, Mosquera JA (2002) Short report: increased level of serum nitric oxide in patients with dengue. Am J Trop Med Hyg 66:762–764. https://doi.org/10.4269/ajtmh.2002.66.762
Espina LM, Valero NJ, Hernández JM, Mosquera JA (2003) Increased apoptosis and expression of tumor necrosis factor-alpha caused by infection of cultured human monocytes with dengue virus. Am J Trop Med Hyg 68:48–53
Sadoshima J (2000) Cytokine actions of angiotensin II. Circ Res 86:1187–1189. https://doi.org/10.1161/01.res.86.12.1187
Ruiz-Ortega M, Ruperez M, Lorenzo O, Esteban V, Blanco J, Mezzano S, Egido J (2002) Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int Suppl 82:S12–22. https://doi.org/10.1046/j.1523-1755.62.s82.4.x
Reilly CF, Tewksbury DA, Schechter NM, Travis J (1982) Rapid conversion of angiotensin I to angiotensin II by neutrophil and mast cell proteinases. J Biol Chem 257:8619–8622
Urata H, Nishimura H, Ganten D (1996) Chymase-dependent angiotensin II forming systems in humans. Am J Hypertens 9:277–284. https://doi.org/10.1016/0895-7061(95)00349-5
Kunder CA, St John AL, Abraham SN (2011) Mast cell modulation of the vascular and lymphatic endothelium. Blood 118:5383–5393. https://doi.org/10.1182/blood-2011-07-358432
Sherif NA, Zayan AH, Elkady AH, Ghozy S, Ahmed AR, Omran ES, Taha EA, Eldesoky EA, Ebied A, Tieu T, Maraie N, Kamel MG, Ngo HT, Mattar OM, Hirayama K, Huy NT (2020) Mast cell mediators in relation to dengue severity: A systematic review and meta-analysis. Rev Med Virol 30:e2084. https://doi.org/10.1002/rmv.2084
Ahmad S, Varagic J, Groban L, Dell’Italia LJ, Nagata S, Kon ND, Ferrario CM (2014) Angiotensin-(1–12): a chymase-mediated cellular angiotensin II substrate. Curr Hypertens Rep 16:429. https://doi.org/10.1007/s11906-014-0429-9
Miyazaki M, Takai S (2006) Tissue angiotensin II generating system by angiotensin-converting enzyme and chymase. J Pharmacol Sci 100:391–397. https://doi.org/10.1254/jphs.cpj06008x
Aloia AL, Abraham AM, Bonder CS, Pitson SM, Carr JM (2015) Dengue Virus-Induced Inflammation of the Endothelium and the Potential Roles of Sphingosine Kinase-1 and MicroRNAs. Mediators Inflamm 2015:509306. https://doi.org/10.1155/2015/509306
Lee RC, Feinbaum RL, Ambros V (1993) The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75:843–854. https://doi.org/10.1016/0092-8674(93)90529-y
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297. https://doi.org/10.1016/s0092-8674(04)00045-5
Jeppesen PL, Christensen GL, Schneider M, Nossent AY, Jensen HB, Andersen DC, Eskildsen T, Gammeltoft S, Hansen JL, n Sheikh SP (2011) Angiotensin II type 1 receptor signalling regulates microRNA differentially in cardiac fibroblasts and myocytes. Br J Pharmacol 164:394–404. https://doi.org/10.1111/j.1476-5381.2011.01375.x
Song J, Yang M, Liu Y, Song J, Wang J, Chi H, Liu X, Zuo K, Yang X, Zhong J (2020) MicroRNA-122 aggravates angiotensin II-mediated apoptosis and autophagy imbalance in rat aortic adventitial fibroblasts via the modulation of SIRT6-elabela-ACE2 signaling. Eur J Pharmacol 883:173374. https://doi.org/10.1016/j.ejphar.2020.173374
Kansakar U, Gambardella J, Varzideh F, Avvisato R, Jankauskas SS, Mone P, Matarese A, Santulli G (2022) miR-142 Targets TIM-1 in Human Endothelial Cells: Potential Implications for Stroke, COVID-19, Zika, Ebola, Dengue, and Other Viral Infections. Int J Mol Sci 23:10242. https://doi.org/10.3390/ijms231810242
Chu LW, Chia-Jui Yang CJ, Kuan-Jen Peng KJ, Pei-Ling Chen PL, Shuu-Jiun Wang SJ, Yueh-Hsin P (2019) TIM-1 As a Signal Receptor Triggers Dengue Virus-Induced Autophagy. Int J Mol Sci 20:4893. https://doi.org/10.3390/ijms20194893
Dejarnac O, Hafirassou ML, Chazal M, Versapuech M, Gaillard J, Perera-Lecoin M, Umana-Diaz C, Bonnet-Madin L, Carnec X, Tinevez JY, Delaugerre C, Schwartz O, Roingeard P, Jouvenet N, Berlioz-Torrent C, Meertens L, Amara A (2018) TIM-1 Ubiquitination Mediates Dengue Virus Entry. Cell Rep 23:1779–1793. https://doi.org/10.1016/j.celrep.2018.04.013
Adamcova M, Kawano I, Simko F (2021) The Impact of microRNAs in Renin–Angiotensin-SystemInduced Cardiac Remodelling. Int J Mol Sci 22:4762. https://doi.org/10.3390/ijms22094762
Sharma S (2017) Immunomodulation: a definitive role of microRNA-142. Dev Comp Immunol 77:150–156. https://doi.org/10.1016/j.dci.2017.08.001
Gupta N, Shweta Jadhav S, Kai-Leng Tan KL, Genevieve Saw G, Karthik Babu Mallilankaraman KB, Dheen ST (2020) miR-142-3p Regulates BDNF Expression in Activated Rodent Microglia Through Its Target CAMK2A. Front Cell Neurosci 14:132. https://doi.org/10.3389/fncel.2020.00132
Qin B, Shu Y, Long L, Li H, Men X, Feng L, Yang H, Lu Z (2018) MicroRNA-142-3p Induces Atherosclerosis-Associated Endothelial Cell Apoptosis by Directly Targeting Rictor. Cell Physiol Biochem 47:1589–1603. https://doi.org/10.1159/000490932
Michel FS, Norton GR, Maseko MJ, Majane OH, Sareli P, Woodiwiss AJ (2014) Urinary Angiotensinogen Excretion Is Associated With Blood Pressure Independent of the Circulating Renin–Angiotensin System in a Group of African Ancestry. Hypertension 64:149–156. https://doi.org/10.1161/HYPERTENSIONAHA.114.03336. PMID: 24777983
Nhi DM, Huy NT, Ohyama K, Kimura D, Lan NTP, Uchida L (2016) A Proteomic Approach Identifies Candidate Early Biomarkers to Predict Severe Dengue in Children. PLoS Negl Trop Dis 10:e0004435. https://doi.org/10.1371/journal.pntd.0004435
Liew KJ, Chow VT (2006) Microarray and real-time RT-PCR analyses of a novel set of differentially expressed human genes in ECV304 endothelial-like cells infected with dengue virus type 2. J Virol Methods 131:47–57. https://doi.org/10.1016/j.jviromet.2005.07.003
Malavige GN, Ranatunga PK, Velathanthiri VG, Fernando S, Karunatilaka DH, Aaskov J, Seneviratne SL (2006) Patterns of disease in Sri Lankan dengue patients. Arch Dis Child 91:396–400. https://doi.org/10.1136/adc.2005.085191
Giani JF, Janjulia T, Taylor B, Bernstein EA, Shah K, Shen XZ, McDonough AA, Bernstein KE, Gonzalez-Villalobos RA (2014) Renal generation of angiotensin II and the pathogenesis of hypertension. Curr Hypertens Rep 16:477. https://doi.org/10.1007/s11906-014-0477-1
Shyu HW, Lin YY, Chen LC, WangYF, Yeh TM, Su SJ, Cheng WC, Chen CY, Lin KH, Chou MC (2010) The dengue virus envelope protein induced PAI-1 gene expression via MEK/ERK pathways. Thromb Haemost 104:1219–1227. https://doi.org/10.1160/TH10-05-0302
Menzel N, Fischl W, Hueging K, Bankwitz D, Frentzen A, Haid S, Pietschmann T (2012) MAP-kinase regulated cytosolic phospholipase A2 activity is essential for production of infectious hepatitis C virus particles. PLoS Pathog 8:e1002829. https://doi.org/10.1371/journal.ppat.1002829
de Oliveira LC, Ribeiro AM, Torres JD, Guimarães AA, Pinto LFZ, Parker AK, Doronin S, Brien K, Buller JD, Bonjardim MR CA (2020) The small molecule AZD6244 inhibits dengue virus replication in vitro and protects against lethal challenge in a mouse model. Arch Virol 165:671–681. https://doi.org/10.1007/s00705-020-04524-7
Lu Y, Sun X, Peng L, Jiang W, Li W, Yuan H, Cai J (2020) Angiotensin II-Induced vascular remodeling and hypertension involves cathepsin L/V- MEK/ERK mediated mechanism. Int J Cardiol 298:98–106. https://doi.org/10.1016/j.ijcard.2019.09.070
Takeda K, Ichiki T, Tokunou T, Iino N, Fujii S, Kitabatake A, Shimokawa H, Takeshita A (2001) Critical role of Rho-kinase and MEK/ERK pathways for angiotensin II-induced plasminogen activator inhibitor type-1 gene expression. Arterioscler Thromb Vasc Biol 21:868–873. https://doi.org/10.1161/01.atv.21.5.868
Maddahi A, Edvinsson L (2010) Cerebral ischemia induces microvascular pro-inflammatory cytokine expression via the MEK/ERK pathway. J Neuroinflammation 7:14. https://doi.org/10.1186/1742-2094-7-14
Albarnaz JD, De Oliveira LC, Torres AA, Palhares RM, Casteluber MC, Rodrigues CM, Cardozo PL, De Souza AMR, Pacca CC, Ferreira PCP, Kroon EG, Nogueira ML, Bonjardim CA (2014) MEK/ERK activation plays a decisive role in yellow fever virus replication: implication as an antiviral therapeutic target. Antiviral Res 111:82–92. https://doi.org/10.1016/j.antiviral.2014.09.004
Chuang YC, Lei HY, Liu HS, Lin YS, Fu TF, Yeh TM (2011) Macrophage migration inhibitory factor induced by dengue virus infection increases vascular permeability. Cytokine 54:222–231. https://doi.org/10.1016/j.cyto.2011.01.013
Yang L, Zou X, Liang Q, Chen H, Feng J, Yan L, Wang Z, Zhou D, Li S, Yao S, Zheng Z (2007) Sodium tanshinone IIA sulfonate depresses angiotensin II-induced cardiomyocyte hypertrophy through MEK/ERK pathway. Exp Mol Med 39:65–73. https://doi.org/10.1038/emm.2007.8
Hazan-Halevy I, Levy T, Wolak T, Lubarsky I, Levy R, Paran E (2005) Stimulation of NADPH oxidase by angiotensin II in human neutrophils is mediated by ERK, p38 MAP-kinase and cytosolic phospholipase A2. J Hypertens 23:1183–1190. https://doi.org/10.1097/01.hjh.0000170381.53955.68
Douillette A, Bibeau-Poirier A, Gravel SP, ClémentJF, Chénard V, Moreau P, Servant MJ (2006) The proinflammatory actions of angiotensin II are dependent on p65 phosphorylation by the IkappaB kinase complex. J Biol Chem 281:13275–13284. https://doi.org/10.1074/jbc.M512815200
Chuang FK, Liao CL, Hu MK, Chiu YL, Lee AR, Huang SM, Chiu YL, Tsai PL, Su BC, Chang TH, Lin CC, Shih CC, Yen LC (2020) Antiviral Activity of Compound L3 against Dengue and Zika Viruses In Vitro and In Vivo. Int J Mol Sci 21:4050. https://doi.org/10.3390/ijms21114050
Ahmad SAA, Palanisamy UD, Khoo JJ, Dhanoa A, Hassan SS (2019) Efficacy of geraniin on dengue virus type-2 infected BALB/c mice. Virol J 16:1626. https://doi.org/10.1186/s12985-019-1127-7
Good SS, Shannon A, Lin K, Moussa A, Julander JG, La Colla P, Collu G, Canard B, Sommadossi JP (2021) Evaluation of AT-752, a double prodrug of a guanosine nucleotide analog with in vitro and in vivo activity against dengue and other flaviviruses. Antimicrob Agents Chemother 65:e00988–e00921. https://doi.org/10.1128/AAC.00988-21
Berk BC, Corson MA (1997) Angiotensin II Signal Transduction in Vascular Smooth Muscle Role of Tyrosine Kinases. Circ Res 80:607–616. https://doi.org/10.1161/01.RES.80.5.607
Park EJ, Jhon DY (2010) The antioxidant, angiotensin converting enzyme inhibition activity, and phenolic compounds of bamboo shoot extracts. LWT - Food Science and Technology 43:655–659
Zhang Y, Pechan T, Chang SKC (2018) Antioxidant and angiotensin-I converting enzyme inhibitory activities of phenolic extracts and fractions derived from three phenolic-rich legume varieties. J Funct Foods 42:289–297. https://doi.org/10.1016/j.jff.2017.12.060
Glossmann H, Baukal A, Catt KJ (1974) Angiotensin II receptors in bovine adrenal cortex. Modification of angiotensin II binding by guanyl nucleotides. J Biol Chem 249:664–666
Bottari SP, Taylor V, King IN, Bogdal Y, Whitebread S, de Gasparo M (1991) Angiotensin II AT2 receptors do not interact with guanine nucleotide binding proteins. Eur J Pharmacol Mol Pharmacol 207:157–163
Yang P, Gu H, Zhao Z, Wang W, Cao B, Lai C, Yang X, Zhang L, Duan Y, Zhang S, Chen W, Zhen W, Cai M, Penninger JM, Jiang C, Wang X (2014) Angiotensin-converting enzyme 2(ACE2) mediates influenza H7N9 virus-induced acute lung injury. Sci Rep 4:7027–7032. https://doi.org/10.1038/srep07027
Huang F, Guo J, Zou Z, Liu J, Cao B, Zhang S, Li H, Wang W, Sheng M, Liu S, Pan J, Bao C, Zeng M, Xiao H, Qian G, Hu X, Chen Y, Chen Y, Zhao Y, Liu Q, Zhou H, Zhu J, Gao H, Yang S, Liu X, Zheng S, Yang J, Diao H, Cao H, Wu Y, Zhao M, Tan S, Guo D, Zhao X, Ye Y, Wu W, Xu Y, Penninger JM, Li D, Gao GF, Jiang C, Li L (2014) Angiotensin II plasma levels are linked to disease severity and predict fatal outcomes in H7N9-infected patients. Nat Commun 5:3595. https://doi.org/10.1038/ncomms4595
Aksoy H, Karadag AS, Wollina U (2020) Angiotensin II receptors: Impact for COVID-19 severity. Dermatol Ther 33:e13989. https://doi.org/10.1111/dth.13989
El-Arif G, Farhat A, Khazaal S, Annweiler C, Kovacic H, Wu Y, Cao Z, Fajloun Z, Khattar ZA, Sabatier JM (2021) The Renin-Angiotensin System: A Key Role in SARS-CoV-2-Induced COVID-19. Molecules 26:6945. https://doi.org/10.3390/molecules26226945
Ang LT, Tan LY, Chow VT, Sim MK (2012) Des-aspartateangiotensin I exerts antiviral effects and attenuates ICAM-1 formation in rhinovirus-infected epithelial cells. Eur J Pharmacol 683:310–315. https://doi.org/10.1016/j.ejphar.2012.02.032
Peña C, Hernandez-Fonseca JP, Rincon J, Pedreañez A, Viera N, Mosquera J (2013) Proinflammatory role of angiotensin II in mercuric induced nephropathy in rats. J Immunotoxicol 10:125–132. https://doi.org/10.3109/1547691X.2012.699478
Vargas R, Rincon J, Pedreañez A, Viera N, Hernandez-Fonseca JP, Peña C, Mosquera J (2012) Role of Angiotensin II in the brain inflammatory events during experimental diabetes in rats. Brain Res 1453:64–76. https://doi.org/10.1016/j.brainres.2012.03.021
Muñoz M, Rincon J, Pedreañez A, Viera N, Hernandez-Fonseca JP, Mosquera J (2011) Proinflammatory role of angiotensin II in a rat nephrosis model induced by adriamycin. J Renin Angiotensin Aldosterone Syst 12:404–412. https://doi.org/10.1177/1470320311410092
Acknowledgements:
We thank Instituto de Investigaciones Clínicas Dr. Américo Negrette, Facultad de Medicina, Universidad de Zulia, Maracaibo, Venezuela.
Funding
This study did not receive any financial support.
Author information
Authors and Affiliations
Contributions
JM-S and AP conceived the subject matter and contributed to the design of the work. JM-S, AP, JPH-F, and HH-F contributed to the acquisition, analysis, or interpretation of data for the work. JM-S and AP wrote the original draft. JM-S, AP, JPH-F, and HH-F critically revised the first draft. All authors approved the final version for all aspects of the work, ensuring integrity and accuracy.
Corresponding author
Ethics declarations
Conflict of interest:
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Communicated by Patricia Aguilar
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mosquera-Sulbaran, J.A., Pedreañez, A., Hernandez-Fonseca, J.P. et al. Angiotensin II and dengue. Arch Virol 168, 191 (2023). https://doi.org/10.1007/s00705-023-05814-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00705-023-05814-6