Abstract
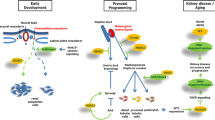
During the early stages of the development of the living multiorgan systems, genome modifications other than sequence variation occur that guide cell differentiation and organogenesis. These modifications are known to operate as a fetal programming code during this period, and recent research indicates that there are some tissue-specific codes in organogenesis whose effects may persist after birth until adulthood. Consequently, the events that disrupt the pre-established epigenetic pattern could induce shifts in organ physiology, with implications on health from birth or later in adult life. Chronic kidney disease (CKD) is one of the main causes of mortality worldwide; its etiology is multifactorial, but diabetes, obesity, and hypertension are the main causes of CKD in adults, although there are other risk factors that are mainly associated with an individual’s lifestyle. Recent studies suggest that fetal reprogramming in the developing kidney could be implicated in the susceptibility to kidney disease in both childhood and adulthood. Some epigenetic modifications, such as genome methylation status, dysregulation of miRNA, and histone coding alterations in genes related to the regulation of the renin-angiotensin axis, a common denominator in CKD, may have originated during fetal development. This review focuses on epigenetic changes during nephrogenesis and their repercussions on kidney health and disease. In addition, the focus is on the influence of environmental factors during pregnancy, such as maternal metabolic diseases and dietary and metabolic conditions, as well as some sex differences in fetal kidney reprogramming during which dysregulation of the renin-angiotensin system is involved.


Similar content being viewed by others
References
Harambat J, Van Stralen KJ, Kim JJ, Tizard EJ (2012) Epidemiology of chronic kidney disease in children. Pediatr Nephrol 27:363–373. https://doi.org/10.1007/s00467-011-1939-1
Simeoni U, Armengaud JB, Siddeek B, Tolsa JF (2018) Perinatal origins of adult disease. Neonatology 113:393–399. https://doi.org/10.1159/000487618
Morrison JL, Ayonrinde OT, Care AS, Clarke GD, Darby JRT, David AL, Dean JM, Hooper SB, Kitchen MJ, Macgowan CK, Melbourne A, McGillick EV, McKenzie CA, Michael N, Mohammed N, Sadananthan SA, Schrauben E, Regnault TRH, Velan SS (2021) Seeing the fetus from a DOHaD perspective: discussion paper from the advanced imaging techniques of DOHaD applications workshop held at the 2019 DOHaD World Congress. J Dev Orig Health Dis 12:153–167. https://doi.org/10.1017/S2040174420000884
Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE (2011) Human nephron number: implications for health and disease. Pediatr Nephrol 26:1529–1533. https://doi.org/10.1007/s00467-011-1843-8
Ryan D, Sutherland MR, Flores TJ, Kent AL, Dahlstrom JE, Puelles VG, Bertram JF, McMahon AP, Little MH, Moore L, Black MJ (2018) Development of the human fetal kidney from mid to late gestation in male and female infants. EBioMedicine 27:275–283. https://doi.org/10.1016/j.ebiom.2017.12.016
Barker DJ (1998) In utero programming of chronic disease. Clin Sci (Lond) 95:115–128
Sarkies P (2020) Molecular mechanisms of epigenetic inheritance: possible evolutionary implications. Semin Cell Dev Biol 97:106–115. https://doi.org/10.1016/j.semcdb.2019.06.005
Law PP, Holland ML (2019) DNA methylation at the crossroads of gene and environment interactions. Essays Biochem 63:717–726. https://doi.org/10.1042/EBC20190031
McLaughlin N, Wang F, Saifudeen Z, El-Dahr SS (2014) In situ histone landscape of nephrogenesis. Epigenetics 9:222–235. https://doi.org/10.4161/epi.26793
Franczyk B, Gluba-Brzozka A, Olszewski R, Parolczyk M, Rysz-Gorzynska M, Rysz J (2022) miRNA biomarkers in renal disease. Int Urol Nephrol 54:575–588. https://doi.org/10.1007/s11255-021-02922-7
Guan Y, Liu H, Ma Z, Li SY, Park J, Sheng X, Susztak K (2020) Dnmt3a and Dnmt3b-decommissioned fetal enhancers are linked to kidney disease. J Am Soc Nephrol 31:765–782. https://doi.org/10.1681/ASN.2019080797
Chu AY, Tin A, Schlosser P, Ko YA, Qiu C, Yao C, Joehanes R, Grams ME, Liang L, Gluck CA, Liu C, Coresh J, Hwang SJ, Levy D, Boerwinkle E, Pankow JS, Yang Q, Fornage M, Fox CS, Susztak K, Köttgen A (2017) Epigenome-wide association studies identify DNA methylation associated with kidney function. Nat Commun 8:1286. https://doi.org/10.1038/s41467-017-01297-7
Takimoto-Ohnishi E, Murakami K (2019) Renin-angiotensin system research: from molecules to the whole body. J Physiol Sci 69:581–587. https://doi.org/10.1007/s12576-019-00679-4
Elgazzaz M, Lazartigues E (2021) Epigenetic modifications of the renin-angiotensin system in cardiometabolic diseases. Clin Sci (Lond) 135:127–142. https://doi.org/10.1042/CS20201287
Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R (2001) Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res 49:460–467. https://doi.org/10.1203/00006450-200104000-00005
Bogdarina I, Welham S, King PJ, Burns SP, Clark AJ (2007) Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ Res 100:520–526. https://doi.org/10.1161/01.RES.0000258855.60637.58
Kawakami-Mori F, Nishimoto M, Reheman L, Kawarazaki W, Ayuzawa N, Ueda K, Hirohama D, Kohno D, Oba S, Shimosawa T, Marumo T, Fujita T (2018) Aberrant DNA methylation of hypothalamic angiotensin receptor in prenatal programmed hypertension. JCI Insight 3:21. https://doi.org/10.1172/jci.insight.95625
Gobetto MN, Mendes GarridoAbregú F, Caniffi C, Veiras L, Elesgaray R, Gironacci M, Tomat AL, Arranz C (2020) Fetal and postnatal zinc restriction: sex differences in the renal renin-angiotensin system of newborn and adult Wistar rats. J Nutr Biochem 81:108385. https://doi.org/10.1016/j.jnutbio.2020.108385
South AM, Shaltout HA, Washburn LK, Hendricks AS, Diz DI, Chappell MC (2019) Fetal programming and the angiotensin-(1–7) axis: a review of the experimental and clinical data. Clin Sci (Lond) 133:55–74. https://doi.org/10.1042/CS20171550
Inoue I, Nakajima T, Williams CS, Quackenbush J, Puryear R, Powers M, Cheng T, Ludwig EH, Sharma AM, Hata A, Jeunemaitre X, Lalouel JM (1997) A nucleotide substitution in the promoter of human angiotensinogen is associated with essential hypertension and affects basal transcription in vitro. J Clin Invest 99:1786–1797. https://doi.org/10.1172/JCI119343
DuPriest E, Hebert J, Morita M, Marek N, Meserve EEK, Andeen N, Houseman EA, Qi Y, Alwasel S, Nyengaard J, Morgan T (2020) Fetal renal DNA methylation and developmental programming of stress-induced hypertension in growth-restricted male mice. Reprod Sci 27:1110–1120. https://doi.org/10.1007/s43032-019-00121-5
Ajala AR, Almeida SS, Rangel M, Palomino Z, Strufaldi MW, Puccini RF, Araujo RC, Casarini DE, Franco MC (2012) Association of ACE gene insertion/deletion polymorphism with birth weight, blood pressure levels, and ACE activity in healthy children. Am J Hypertens 25:827–832. https://doi.org/10.1038/ajh.2012.50
He Q, Fan C, Yu M, Wallar G, Zhang ZF, Wang L, Zhang X, Hu R (2013) Associations of ACE gene insertion/deletion polymorphism, ACE activity, and ACE mRNA expression with hypertension in a Chinese population. PLoS One 1:e75870. https://doi.org/10.1371/journal.pone.0075870
Acevedo N, Alashkar Alhamwe B, Caraballo L, Ding M, Ferrante A, Garn H, Garssen J, Hii CS, Irvine J, Llinás-Caballero K, López JF, Miethe S, Perveen K, Pogge von Strandmann E, Sokolowska M, Potaczek DP, van Esch BCAM (2021) Perinatal and early-life nutrition, epigenetics, and allergy. Nutrients 25:724. https://doi.org/10.3390/nu13030724
Zheng J, Zhang L, Liu J, Li Y, Zhang J (2021) Long-term effects of maternal low-protein diet and post-weaning high-fat feeding on glucose metabolism and hypothalamic POMC promoter methylation in offspring mice. Front Nutr 16:657848. https://doi.org/10.3389/fnut.2021.657848
Winship AL, Gazzard SE, Cullen-McEwen LA, Bertram JF, Hutt KJ (2018) Maternal low-protein diet programmes low ovarian reserve in offspring. Reproduction 156:299–311. https://doi.org/10.1530/REP-18-0247
Ajuogu PK, Al-Aqbi MAK, Hart RA, McFarlane JR, Smart NA (2021) A low protein maternal diet during gestation has negative effects on male fertility markers in rats - a systematic review and meta-analysis. J Anim Physiol Anim Nutr (Berl) 105:157–166. https://doi.org/10.1111/jpn.13411
Esmeijer K, de Vries AP, Mook-Kanamori DO, de Fijter JW, Rosendaal FR, Rabelink TJ, Smit RAJ, de Mutsert R, Hoogeveen EK (2019) Low birth weight and kidney function in middle-aged men and women: the Netherlands epidemiology of obesity study. Am J Kidney Dis 74:751–760. https://doi.org/10.1053/j.ajkd.2019.05.007
Grillo MA, Mariani G, Ferraris JR (2021) Prematurity and low weight in neonates as a risk factor for obesity, hypertension, and chronic kidney disease in pediatric and adult age. Front Med (Lausanne) 8:769734. https://doi.org/10.3389/fmed.2021.769734
Rodríguez López S, Tumas N, Ortigoza A, de Lima Friche AA, Diez-Roux AV (2021) Urban social environment and low birth weight in 360 Latin American cities. BMC Public Health 21:795. https://doi.org/10.1186/s12889-021-10886-7
Blencowe H, Krasevec J, de Onis M, Black RE, An X, Stevens GA, Borghi E, Hayashi C, Estevez D, Cegolon L, Shiekh S, Ponce Hardy V, Lawn JE, Cousens S (2019) National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: a systematic analysis. Lancet Glob Health 7:e849–e860. https://doi.org/10.1016/S2214-109X(18)30565-5
Liyanage T, Toyama T, Hockham C, Ninomiya T, Perkovic V, Woodward M, Fukagawa M, Matsushita K, Praditpornsilpa K, Hooi LS, Iseki K, Lin MY, Stirnadel-Farrant HA, Jha V, Jun M (2022) Prevalence of chronic kidney disease in Asia: a systematic review and analysis. BMJ Glob Health 7:e007525. https://doi.org/10.1136/bmjgh-2021-007525
Miyoshi M, Sato M, Saito K, Otani L, Shirahige K, Miura F, Ito T, Jia H, Kato H (2018) Maternal protein restriction alters the renal Ptger1 DNA methylation state in SHRSP offspring. Nutrients 10:10. https://doi.org/10.3390/nu10101436
Mao C, Liu R, Bo L, Chen N, Li S, Xia S, Chen J, Li D, Zhang L, Xu Z (2013) High-salt diets during pregnancy affected fetal and offspring renal renin-angiotensin system. J Endocrinol 218:61–73. https://doi.org/10.1530/JOE-13-0139
Tay S, Blache D, Gregg K, Revell D (2012) Consumption of a high-salt diet by ewes during pregnancy alters nephrogenesis in 5-month-old offspring. Animal 6:1803–1810. https://doi.org/10.1017/S1751731112000584
Brito S, Lee MG, Bin BH, Lee JS (2020) Zinc and its transporters in epigenetics. Mol Cells 43:323–330. https://doi.org/10.14348/molcells.2020.0026
Robinson SM, Batelaan SF, Syddall HE, Sayer AA, Dennison EM, Martin HJ, Barker DJ, Cooper C (2006) Combined effects of dietary fat and birth weight on serum cholesterol concentrations: the Hertfordshire Cohort Study. Am J Clin Nutr 84:237–244. https://doi.org/10.1093/ajcn/84.1.237
Simões-Alves AC, Arcoverde-Mello APFC, Campos JO, Wanderley AG, Leandro CVG, da Costa-Silva JH, de Oliveira Nogueira Souza V (2022) Cardiometabolic effects of postnatal high-fat diet consumption in offspring exposed to maternal protein restriction in utero. Front Physiol 10:829920. https://doi.org/10.3389/fphys.2022.829920
Pereira Júnior CD, Guimarães CS, da Silva AC, Rodrigues AR, da Glória MA, Teixeira VP, Câmara NO, Rocha LB, Dos Reis MA, Machado JR, Rocha LP, Helmo FR, Corrêa RR (2016) Influence of the expression of inflammatory markers on kidney after fetal programming in an experimental model of renal failure. J Immunol Res 2016:9151607. https://doi.org/10.1155/2016/9151607
Casasnovas J, Jo Y, Rao X, Xuei X, Brown ME, Kua KL (2019) High glucose alters fetal rat islet transcriptome and induces progeny islet dysfunction. J Endocrinol 240:309–323. https://doi.org/10.1530/JOE-18-0493
Strakovsky RS, Zhang X, Zhou D, Pan YX (2011) Gestational high fat diet programs hepatic phosphoenolpyruvate carboxykinase gene expression and histone modification in neonatal offspring rats. J Physiol 589:2707–2717. https://doi.org/10.1113/jphysiol.2010.203950
Brennan S, Kandasamy Y, Rudd DM, Schneider ME, Jones RE, Watson DL (2020) The effect of diabetes during pregnancy on fetal renal parenchymal growth. J Nephrol 33:1079–1089. https://doi.org/10.1007/s40620-020-00815-z
Abi Khalil C, Travert F, Fetita S, Rouzet F, Porcher R, Riveline JP, Hadjadj S, Larger E, Roussel R, Vexiau P, Le Guludec D, Gautier JF, Marre M (2010) Fetal exposure to maternal type 1 diabetes is associated with renal dysfunction at adult age. Diabetes 59:2631–2636. https://doi.org/10.2337/db10-0419
Chen YW, Chenier I, Tran S, Scotcher M, Chang SY, Zhang SL (2010) Maternal diabetes programs hypertension and kidney injury in offspring. Pediatr Nephrol 25:1319–1329. https://doi.org/10.1007/s00467-010-1506-1
Yan J, Li X, Su R, Zhang K, Yang H (2014) Long-term effects of maternal diabetes on blood pressure and renal function in rat male offspring. PLoS One 9:e88269. https://doi.org/10.1371/journal.pone.0088269
Glastras SJ, Chen H, Teh R, McGrath RT, Chen J, Pollock CA, Wong MG, Saad S (2016) Mouse models of diabetes, obesity and related kidney disease. PLoS One 11:e0162131. https://doi.org/10.1371/journal.pone.0162131
Larkin BP, Glastras SJ, Chen H, Pollock CA, Saad S (2018) DNA methylation and the potential role of demethylating agents in prevention of progressive chronic kidney disease. FASEB J 32:5215–5226. https://doi.org/10.1096/fj.201800205R
Glastras SJ, Chen H, Pollock CA, Saad S (2018) Maternal obesity increases the risk of metabolic disease and impacts renal health in offspring. Biosci Rep 38:2. https://doi.org/10.1042/BSR20180050
Bechtel W, McGoohan S, Zeisberg EM, Müller GA, Kalbacher H, Salant DJ, Müller CA, Kalluri R, Zeisberg M (2010) Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat Med 16:544–550. https://doi.org/10.1038/nm.2135
Larkin BP, Nguyen LT, Hou M, Glastras SJ, Chen H, Wang R, Pollock CA, Saad S (2021) Novel role of gestational hydralazine in limiting maternal and dietary obesity-related chronic kidney disease. Front Cell Dev Biol 9:705263. https://doi.org/10.3389/fcell.2021.705263
Kassab BM, Hussein HH, Mahmoud OM, Abdel-Alrahman G (2019) Effects of insulin and metformin on fetal kidney development of streptozotocin-induced gestational diabetic albino rats. Anat Cell Biol 52:161–175. https://doi.org/10.5115/acb.2019.52.2.161
Hou K, Wu ZX, Chen XY, Wang JQ, Zhang D, Xiao C, Zhu D, Koya JB, Wei L, Li J, Chen ZS (2022) Microbiota in health and diseases. Signal Transduct Target Ther 23:135. https://doi.org/10.1038/s41392-022-00974-4
Addi T, Dou L, Burtey S (2018) Tryptophan-derived uremic toxins and thrombosis in chronic kidney disease. Toxins (Basel) 10:412. https://doi.org/10.3390/toxins10100412
Velasquez MT, Centron P, Barrows I, Dwivedi R, Raj DS (2018) Gut microbiota and cardiovascular uremic toxicities. Toxins (Basel) 10:287. https://doi.org/10.3390/toxins10070287
Jaworska K, Koper M, Ufnal M (2021) Gut microbiota and renin-angiotensin system: a complex interplay at local and systemic levels. Am J Physiol Gastrointest Liver Physiol 321:G355–G366. https://doi.org/10.1152/ajpgi.00099.2021
Dave LA, Hayes M, Montoya CA, Rutherfurd SM, Moughan PJ (2016) Human gut endogenous proteins as a potential source of angiotensin- I-converting enzyme (ACE-I)-, renin inhibitory and antioxidant peptides. Peptides 76:30–44. https://doi.org/10.1016/j.peptides.2015.11.003
Wang L, Zhu Q, Lu A, Liu X, Zhang L, Xu C, Liu X, Li H, Yang T (2017) Sodium butyrate suppresses angiotensin II-induced hypertension by inhibition of renal (pro) renin receptor and intrarenal renin–angiotensinsystem. J Hypertens 35:1899–1908. https://doi.org/10.1097/HJH.0000000000001378
Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, Sigl V, Hanada T, Hanada R, Lipinski S, Wild B, Camargo SMR, Singer D, Richter A, Kuba K, Fukamizu A, Schreiber S, Clevers H, Verrey F, Rosenstiel P, Penninger JM (2012) ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 487:477–481. https://doi.org/10.1038/nature11228
Tain YL, Hsu CN (2022) Hypertension of developmental origins: consideration of gut microbiome in animal models. Biomedicines 10:875. https://doi.org/10.3390/biomedicines10040875
Lu M, Liu YH, Goh HS, Wang JJ, Yong QC, Wang R, Bian JS (2010) Hydrogen sulfide inhibits plasma renin activity. J Am Soc Nephrol 21:993–1002. https://doi.org/10.1681/ASN.2009090949
Feliers D, Lee HJ, Kasinath BS (2016) Hydrogen sulfide in renal physiology and disease. Antioxid Redox Signal 25:720–731. https://doi.org/10.1089/ars.2015.6596
Guo Q, Feng X, Xue H, Teng X, Jin S, Duan X, Xiao L, Wu Y (2017) Maternal renovascular hypertensive rats treatment with hydrogen sulfide increased the methylation of at1b gene in offspring. Am J Hypertens 30:1220–1227. https://doi.org/10.1093/ajh/hpx124
Gomaa EZ (2020) Human gut microbiota/microbiome in health and diseases: a review. Antonie Van Leeuwenhoek 113:2019–2040. https://doi.org/10.1007/s10482-020-01474-7
Dunwoodie SL (2009) The role of hypoxia in development of the mammalian embryo. Dev Cell 17:755–773. https://doi.org/10.1016/j.devcel.2009.11.008
Hemker SL, Cerqueira DM, Bodnar AJ, Cargill KR, Clugston A, Anslow MJ, Sims-Lucas S, Kostka D, Ho J (2020) Deletion of hypoxia-responsive microRNA-210 results in a sex-specific decrease in nephron number. FASEB J 34:5782–5799. https://doi.org/10.1096/fj.201902767R
Cargill KR, Chiba T, Murali A, Mukherjee E, Crinzi E, Sims-Lucas S (2020) Prenatal hypoxia increases susceptibility to kidney injury. PLoS One 15:e0229618. https://doi.org/10.1371/journal.pone.0229618
Lin NW, Liu C, Yang IV, Maier LA, DeMeo DL, Wood C, Ye S, Cruse MH, Smith VL, Vyhlidal CA, Kechris K, Sharma S (2022) Sex-specific differences in microRNA expression during human fetal lung development. Front Genet 13:762834. https://doi.org/10.3389/fgene.2022.762834
Baylis C (2005) Changes in renal hemodynamics and structure in the aging kidney; sexual dimorphism and the nitric oxide system. Exp Gerontol 40:271–278. https://doi.org/10.1016/j.exger.2005.01.008
Loria A, Reverte V, Salazár F, Saez F, Llinas MT, Salazár FJ (2007) Sex and age differences of renal function in rats with reduced ANG II activity during the nephrogenic period. Am J Physiol Renal Physiol 293:F506–F510. https://doi.org/10.1152/ajprenal.00066.2007
Ortiz LA, Quan A, Zarzar F, Weinberg A, Baum M (2003) Prenatal dexamethasone programs hypertension and renal injury in the rat. Hypertension 41:328–334. https://doi.org/10.1161/01.hyp.0000049763.51269.51
Tang L, Bi J, Valego N, Carey L, Figueroa J, Chappell M, Rose JC (2010) Prenatal betamethasone exposure alters renal function in immature sheep: sex differences in effects. Am J Physiol Regul Integr Comp Physiol 299:R793–R7803. https://doi.org/10.1152/ajpregu.00590.2009
Tain YL, Sheen JM, Yu HR, Chen CC, Tiao MM, Hsu CN, Lin YJ, Kuo KC, Huang LT (2015) Maternal melatonin therapy rescues prenatal dexamethasone and postnatal high-fat diet induced programmed hypertension in male rat offspring. Front Physiol 6:377. https://doi.org/10.3389/fphys.2015.00377
Reckelhoff JF, Yanes LL, Iliescu R, Fortepiani LA, Granger JP (2005) Testosterone supplementation in aging men and women: possible impact on cardiovascular-renal disease. Am J Physiol Renal Physiol 289:F941–F948. https://doi.org/10.1152/ajprenal.00034.2005
Yanes LL, Romero DG (2009) Dihydrotestosterone stimulates aldosterone secretion by H295R human adrenocortical cells. Mol Cell Endocrinol 303:50–56. https://doi.org/10.1016/j.mce.2008.12.020
Nalivaeva NN, Zhuravin IA, Turner AJ (2020) Neprilysin expression and functions in development, ageing and disease. Mech Ageing Dev 192:111363. https://doi.org/10.1016/j.mad.2020.111363
Clifton VL, Murphy VE (2004) Maternal asthma as a model for examining fetal sex-specific effects on maternal physiology and placental mechanisms that regulate human fetal growth. Placenta 25(Suppl A):S45–S52. https://doi.org/10.1016/j.placenta.2004.01.004
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pérez-Coria, M., Vázquez-Rivera, G.E., Gómez-García, E.F. et al. Sex differences in fetal kidney reprogramming: the case in the renin-angiotensin system. Pediatr Nephrol 39, 645–653 (2024). https://doi.org/10.1007/s00467-023-06112-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-023-06112-8