Abstract
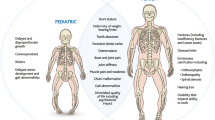
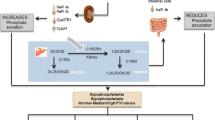
X-linked hypophosphatemia is an X-linked dominant disorder resulting from a mutation in the PHEX gene. PHEX stands for phosphate-regulating gene with endopeptidase activity, which is located on the X chromosome. Patients with X-linked hypophosphatemia have hypophosphatemia due to renal phosphate wasting and low or inappropriately normal levels of 1,25-dihydroxyvitamin D. The renal phosphate wasting is not intrinsic to the kidney but likely due to an increase in serum levels of fibroblast growth factor-23 (FGF-23), and perhaps other phosphate-wasting peptides previously known as phosphatonins. Patients with X-linked hypophosphatemia have short stature, rickets, bone pain and dental abscesses. Current therapy is oral phosphate and vitamin D which effectively treats the rickets and bone pain but does not adequately improve short stature. In this review, we describe recent observations using Hyp mice; mice with the same mutation as patients with X-linked hypophosphatemia. We have recently found that Hyp mice have abnormal renal prostaglandin production, which may be an important factor in the pathogenesis of this disorder. Administration of FGF-23 in vivo results in phosphaturia and an increase in prostaglandin excretion, and FGF-23 increases proximal tubule prostaglandin production in vitro. In Hyp mice, indomethacin improves the phosphate transport defect in vitro and in vivo. Whether indomethacin has the same effect in patients with X-linked hypophosphatemia is unknown.







Similar content being viewed by others
References
Tenenhouse HS, Econs MJ (2001) Mendelian hypophosphatemias. In: Scriver CR, Beaudet AL, Sly WS, Valley D (eds) The metabolic basis of inherited disease. McGraw-Hill, New York, pp 5039–5067
Scriver CR, Reade TM, DeLuca HF, Hamstra AJ (1978) Serum 1,25-dihydroxyvitamin D levels in normal subjects and in patients with hereditary rickets or bone disease. N Engl J Med 299:976–979
Drezner MK, Lyles KW, Haussler MR, Harrelson JM (1980) Evaluation of a role for 1,25-dihydroxyvitamin D3 in the pathogenesis and treatment of X-linked hypophosphatemic rickets and osteomalacia. J Clin Invest 66:1020–1032
Fukase M, Avioli LV, Birge SJ, Chase LR (1984) Abnormal regulation of 25-hydroxyvitamin D3-1 alpha-hydroxylase activity by calcium and calcitonin in renal cortex from hypophosphatemic (Hyp) mice. Endocrinology 114:1203–1207
Tenenhouse HS, Jones G (1990) Abnormal regulation of renal vitamin D catabolism by dietary phosphate in murine X-linked hypophosphatemic rickets. J Clin Invest 85:1450–1455
Verge CF, Lam A, Simpson JM, Cowell CT, Howard NJ, Silink M (1991) Effects of therapy in X-linked hypophosphatemic rickets. N Engl J Med 325:1843–1848
Goodyer PR, Kronick JB, Jequier S, Reade TM, Scriver CR (1987) Nephrocalcinosis and its relationship to treatment of hereditary rickets. J Pediatr 111:700–704
Reusz GS, Latta K, Hoyer PF, Byrd DJ, Ehrich JH, Brodehl J (1990) Evidence suggesting hyperoxaluria as a cause of nephrocalcinosis in phosphate-treated hypophosphataemic rickets. Lancet 335:1240–1243
Rasmussen H, Pechet M, Anast C, Mazur A, Gertner J, Broadus AE (1981) Long-term treatment of familial hypophosphatemic rickets with oral phosphate and 1 alpha-hydroxyvitamin D3. J Pediatr 99:16–25
Seikaly MG, Browne RH, Baum M (1994) The effect of phosphate supplementation on linear growth in children with X-linked hypophosphatemia. Pediatrics 94:478–481
Seikaly M, Browne R, Baum M (1996) Nephrocalcinosis is associated with renal tubular acidosis in children with X-linked hypophosphatemia. Pediatrics 97:91–93
Beck L, Soumounou Y, Martel J, Krishnamurthy G, Gauthier C, Goodyer CG, Tenenhouse HS (1997) Pex/PEX tissue distribution and evidence for a deletion in the 3′ region of the Pex gene in X-linked hypophosphatemic mice. J Clin Invest 99:1200–1209
Holm IA, Huang X, Kunkel LM (1997) Mutational analysis of the PEX gene in patients with X-linked hypophosphatemic rickets. Am J Hum Genet 60:790–797
Francis F, Hennig S, Korn B, Reinhardt R, de Jong P, Poustka A, Lehrach H, Rowe PSN, Goulding JN, Summerfield T, Mountford R, Read AP, Popowska E, Pronicka E, Davies KE, O’Riordan JLH, Econs MJ, Nesbitt T, Drezner MK, Oudet C, Pannetier S, Hanauer A, Strom TM, Meindl A, Lorenz B, Cagnoli B, Mohnike KL, Murken J, Meitinger T (1995) A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat Genet 11:130–136
Rowe PS, Oudet CL, Francis F, Sinding C, Pannetier S, Econs MJ, Strom TM, Meitinger T, Garabedian M, David A, Macher MA, Questiaux E, Popowska E, Pronicka E, Read AP, Mokrzycki A, Glorieux FH, Drezner MK, Hanauer A, Lehrach H, Goulding JN, O’Riordan JL (1997) Distribution of mutations in the PEX gene in families with X-linked hypophosphataemic rickets (HYP). Hum Mol Genet 6:539–549
Du L, Desbarats M, Viel J, Glorieux FH, Cawthorn C, Ecarot B (1996) cDNA cloning of the murine Pex gene implicated in X-linked hypophosphatemia and evidence for expression in bone. Genomics 36:22–28
Guo R, Quarles LD (1997) Cloning and sequencing of human PEX from a bone cDNA library: evidence for its developmental stage-specific regulation in osteoblasts. J Bone Miner Res 12:1009–1017
Magagnin S, Werner A, Markovich D, Sorribas V, Stange G, Biber J, Murer H (1993) Expression cloning of human and rat renal cortex Na/Pi cotransport. Proc Natl Acad Sci USA 90:5979–5983
Tenenhouse HS, Beck L (1996) Renal Na(+)-phosphate cotransporter gene expression in X-linked Hyp and Gy mice. Kidney Int 49:1027–1032
Collins JF, Ghishan FK (1994) Molecular cloning, functional expression, tissue distribution, and in situ hybridization of the renal sodium phosphate (Na+/P(i)) transporter in the control and hypophosphatemic mouse. FASEB J 8:862–868
Tenenhouse HS, Klugerman AH, Neal JL (1989) Effect of phosphonoformic acid, dietary phosphate and the Hyp mutation on kinetically distinct phosphate transport processes in mouse kidney. Biochim Biophys Acta 984:207–213
Nakagawa N, Arab N, Ghishan FK (1991) Characterization of the defect in the Na(+)-phosphate transporter in vitamin D-resistant hypophosphatemic mice. J Biol Chem 266:13616–13620
Baum M, Moe OW, Zhang J, Dwarakanath V, Quigley R (2005) Phosphatonin washout in Hyp mice proximal tubules: evidence for posttranscriptional regulation 2. Am J Physiol Renal Physiol 288:F363–F370
Lobaugh B, Drezner MK (1983) Abnormal regulation of renal 25-hydroxyvitamin D-1 alpha-hydroxylase activity in the X-linked hypophosphatemic mouse. J Clin Invest 71:400–403
Tenenhouse HS (1984) Investigation of the mechanism for abnormal renal 25-hydroxyvitamin D3- 1-hydroxylase activity in the X-linked Hyp mouse. Endocrinology 115:634–639
Fraser D, Kooh SW, Kind HP, Holick MF, Tanaka Y, DeLuca HF (1973) Pathogenesis of hereditary vitamin-D-dependent rickets. An inborn error of vitamin D metabolism involving defective conversion of 25-hydroxyvitamin D to 1-α,25-dihydroxyvitamin D. N Engl J Med 289:817–822
Collins JF, Scheving LA, Ghishan FK (1995) Decreased transcription of the sodium-phosphate transporter gene in the hypophosphatemic mouse. Am J Physiol 269:F439–F448
Tenenhouse HS, Werner A, Biber J, Ma S, Martel J, Roy S, Murer H (1994) Renal Na(+)-phosphate cotransport in murine X-linked hypophosphatemic rickets. Molecular characterization. J Clin Invest 93:671–676
Meyer RA Jr, Meyer MH, Gray RW (1989) Parabiosis suggests a humoral factor is involved in X-linked hypophosphatemia in mice. J Bone Miner Res 4:493–500
Meyer RA Jr, Tenenhouse HS, Meyer MH, Klugerman AH (1989) The renal phosphate transport defect in normal mice parabiosed to X- linked hypophosphatemic mice persists after parathyroidectomy. J Bone Miner Res 4:523–532
Nesbitt T, Coffman TM, Griffiths R, Drezner MK (1992) Crosstransplantation of kidneys in normal and Hyp mice. Evidence that the Hyp mouse phenotype is unrelated to an intrinsic renal defect. J Clin Invest 89:1453–1459
Nesbitt T, Econs MJ, Byun JK, Martel J, Tenenhouse HS, Drezner MK (1995) Phosphate transport in immortalized cell cultures from the renal proximal tubule of normal and Hyp mice: evidence that the HYP gene locus product is an extrarenal factor. J Bone Miner Res 10:1327–1333
Nesbitt T, Byun JK, Drezner MK (1996) Normal phosphate transport in cells from the S2 and S3 segments of Hyp-mouse proximal renal tubules. Endocrinology 137:943–948
Lajeunesse D, Meyer RA Jr, Hamel L (1996) Direct demonstration of a humorally-mediated inhibition of renal phosphate transport in the Hyp mouse. Kidney Int 50:1531–1538
de Beur SMJ, Finnegan RB, Vassiliadis J, Cook B, Barberio D, Estes S, Manavalan P, Petroziello J, Madden SL, Cho JY, Kumar R, Levine MA, Schiavi SC (2002) Tumors associated with oncogenic osteomalacia express genes important in bone and mineral metabolism. J Bone Miner Res 17:1102–1110
Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T (2001) Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA 98:6500–6505
Rowe PSN, de Zoysa PA, Dong R, Wang HR, White KE, Econs MJ, Oudet CL (2000) MEPE, a new gene expressed in bone marrow and tumors causing osteomalacia. Genomics 67:54–68
Berndt T, Craig TA, Bowe AE, Vassiliadis J, Reczek D, Finnegan R, de Beur SMJ, Schiavi SC, Kumar R (2003) Secreted frizzled-related protein 4 is a potent tumor-derived phosphaturic agent. J Clin Invest 112:785–794
Quarles LD (2003) Evidence for a bone-kidney axis regulating phosphate homeostasis. J Clin Invest 112:642–646
Bresler D, Bruder J, Mohnike K, Fraser WD, Rowe PS (2004) Serum MEPE-ASARM-peptides are elevated in X-linked rickets (HYP): implications for phosphaturia and rickets. J Endocrinol 183:R1–R9
Rowe PS, Kumagai Y, Gutierrez G, Garrett IR, Blacher R, Rosen D, Cundy J, Navvab S, Chen D, Drezner MK, Quarles LD, Mundy GR (2004) MEPE has the properties of an osteoblastic phosphatonin and minhibin. Bone 34:303–319
Campos M, Couture C, Hirata IY, Juliano MA, Loisel TP, Crine P, Juliano L, Boileau G, Carmona AK (2003) Human recombinant endopeptidase PHEX has a strict S1′ specificity for acidic residues and cleaves peptides derived from fibroblast growth factor-23 and matrix extracellular phosphoglycoprotein. Biochem J 373:271–279
Bowe AE, Finnegan R, Jan de Beur SM, Cho J, Levine MA, Kumar R, Schiavi SC (2001) FGF-23 inhibits renal tubular phosphate transport and is a PHEX substrate. Biochem Biophys Res Commun 284:977–981
Guo R, Rowe PSN, Liu S, Quarles LD (2002) Inhibition of proteolytic cleavage of MEPE by Phex. Biochem Biophys Res Commun 297:38–45
Liu S, Brown TA, Zhou J, Xiao ZS, Awad H, Guilak F, Quarles LD (2005) Role of matrix extracellular phosphoglycoprotein in the pathogenesis of X-linked hypophosphatemia. J Am Soc Nephrol 16:1645–1653
Gowen LC, Petersen DN, Mansolf AL, Qi H, Stock JL, Tkalcevic GT, Simmons HA, Crawford DT, Chidsey-Frink KL, Ke HZ, McNeish JD, Brown TA (2003) Targeted disruption of the osteoblast/osteocyte factor 45 gene (OF45) results in increased bone formation and bone mass. J Biol Chem 278:1998–2007
Jonsson KB, Zahradnik R, Larsson T, White KE, Hampson G, Miyauchi A, Ljunggren O, Koshiyama H, Sugimoto T, Oba K, Yamamoto T, Imanishi Y, Econs M, Lavigne J, Jueppner H (2002) FGF-23 is a circulating factor that is elevated in oncogenic osteomalacia and X-linked hypophosphatemic rickets. J Bone Miner Res 17:S158
Yamazaki Y, Shibata M, Okazaki R, Takeuchi Y, Fujita T, Yamashita T, Fukumoto S (2002) FGF-23 protein is present in normal plasma and is increased in patients with tumor-induced osteomalacia. J Bone Miner Res 17:S159
Weber TJ, Liu SG, Indridason OS, Quarles LD (2003) Serum FGF23 levels in normal and disordered phosphorus homeostasis. J Bone Miner Res 18:1227–1234
ADHR Consortium (2000) Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 26:345–348
White KE, Jonsson KB, Carn G, Hampson G, Spector TD, Mannstadt M, Lorenz-Depiereux B, Miyauchi A, Yang IM, Ljunggren O, Meitinger T, Strom TM, Juppner H, Econs MJ (2001) The autosomal dominant hypophosphatemic rickets (ADHR) gene is a secreted polypeptide overexpressed by tumors that cause phosphate wasting. J Clin Endocrinol Metab 86:497–500
Shimada T, Muto T, Urakawa I, Yoneya T, Yamazaki Y, Okawa K, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T (2002) Mutant FGF-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology 143:3179–3182
White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ (2001) Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int 60:2079–2086
de Beur SMJ, Bowe AE, Cho JY, Finnegan R, Kumar R, Levine MA, Schiavi SC (2001) Demonstration that FGF-23, a factor produced by tumors associated with phosphate wasting, inhibits phosphate transport in vitro. J Bone Miner Res 16:S151
John MR, Wickert H, Zaar K, Jonsson KB, Grauer A, Ruppersberger P, Schmidt-Gayk H, Murer H, Ziegler R, Blind E (2001) A case of neuroendocrine oncogenic osteomalacia associated with a PHEX and fibroblast growth factor-23 expressing sinusidal malignant schwannoma. Bone 29:393–402
Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB (2003) Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int 64:2272–2279
Yamashita T, Konishi M, Miyake A, Inui K, Itoh N (2002) Fibroblast growth factor (FGF)-23 inhibits renal phosphate reabsorption by activation of the mitogen-activated protein kinase pathway. J Biol Chem 277:28265–28270
Baum M, Schiavi S, Dwarakanath V, Quigley R (2005) Effect of fibroblast growth factor-23 on phosphate transport in proximal tubules. Kidney Int 68:1148–1153
Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T (2004) FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19:429–435
Guo R, Liu SG, Spurney RF, Quarles LD (2001) Analysis of recombinant Phex: an endopeptidase in search of a substrate. Am J Physiol Endocrinol Metab 281:E837–E847
Liu S, Guo R, Simpson LG, Xiao ZS, Burnham CE, Quarles LD (2003) Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J Biol Chem 278:37419–37426
Larsson T, Marsell R, Schipani E, Ohlsson C, Ljunggren O, Tenenhouse HS, Juppner H, Jonsson KB (2004) Transgenic mice expressing Fibroblast Growth Factor 23 under the control of the α1(I) collagen promoter exhibit growth retardation, osteomalacia and disturbed phosphate homeostasis. Endocrinology 145:3087–3094
Shimada T, Urakawa I, Yamazaki Y, Hasegawa H, Hino R, Yoneya T, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T (2004) FGF-23 transgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type IIa. Biochem Biophys Res Commun 314:409–414
Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T (2004) Targeted ablation of FGF23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113:561–568
Sitara D, Razzaque MS, Hesse M, Yoganathan S, Taguchi T, Erben RG, Juppner H, Lanske B (2004) Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol 23:421–432
Baum M, Loleh S, Saini N, Seikaly M, Dwarakanath V, Quigley R (2003) Correction of proximal tubule phosphate transport defect in Hyp mice in vivo and in vitro with indomethacin. Proc Natl Acad Sci USA 100:11098–11103
Syal A, Schiavi S, Chakravarty S, Dwarakanath V, Quigley R, Baum M (2006) Fibroblast Growth Factor-23 increases mouse PGE2 production in vivo and in vitro. Am J Physiol Renal Physiol 290:F450–F455
Aiken JW, Vane JR (1973) Intrarenal prostaglandin release attenuates the renal vasoconstrictor activity of angiotensin. J Pharmacol Exp Ther 184:678–687
Dunn MJ, Hood VL (1977) Prostaglandins and the kidney. Am J Physiol 233:169–184
Farman N, Pradelles P, Bonvalet JP (1986) Determination of prostaglandin E2 synthesis along rabbit nephron by enzyme immunoassay. Am J Physiol 251:F238–F244
Imbert-Teboul M, Siaume S, Morel F (1986) Sites of prostaglandin E2 (PGE2) synthesis along the rabbit nephron. Mol Cell Endocrinol 45:1–10
Farman N, Pradelles P, Bonvalet JP (1987) PGE2, PGF2 alpha, 6-keto-PGF1 alpha, and TxB2 synthesis along the rabbit nephron. Am J Physiol 252:F53–F59
Takemoto F, Miyanoshita A, Shimamura K, Sunano S, Endou H (1990) Intranephron PGE2 production in stroke-prone spontaneously hypertensive rats. Am J Physiol 258:H987–H993
Dominguez JH, Pitts TO, Brown T, Puschett DB, Schuler F, Chen TC, Puschett JB (1984) Prostaglandin E2 and parathyroid hormone: comparisons of their actions on the rabbit proximal tubule. Kidney Int 26:404–410
Acknowledgements
This work was supported by National Institute of Diabetes and Digestive and Kidney Disease Grant DK-41612 and DK-065842 (to M. Baum).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baum, M., Syal, A., Quigley, R. et al. Role of prostaglandins in the pathogenesis of X-linked hypophosphatemia. Pediatr Nephrol 21, 1067–1074 (2006). https://doi.org/10.1007/s00467-006-0126-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-006-0126-2