Abstract
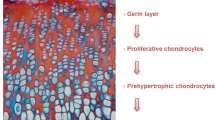
X-Linked hypophosphatemia (XLH) is the most common form of hereditary rickets caused by loss-of function mutations in the PHEX gene. XLH is characterized by hypophosphatemia secondary to renal phosphate wasting, inappropriately low concentrations of 1,25 dihydroxyvitamin D and high circulating levels of fibroblast growth factor 23 (FGF23). Short stature and rachitic osseous lesions are characteristic phenotypic findings of XLH although the severity of these manifestations is highly variable among patients. The degree of growth impairment is not dependent on the magnitude of hypophosphatemia or the extent of legs´ bowing and height is not normalized by chronic administration of phosphate supplements and 1α hydroxyvitamin D derivatives. Treatment with growth hormone accelerates longitudinal growth rate but there is still controversy regarding the potential risk of increasing bone deformities and body disproportion. Treatments aimed at blocking FGF23 action are promising, but information is lacking on the consequences of counteracting FGF23 during the growing period. This review summarizes current knowledge on phosphorus metabolism in XLH, presents updated information on XLH and growth, including the effects of FGF23 on epiphyseal growth plate of the Hyp mouse, an animal model of the disease, and discusses growth hormone and novel FGF23 related therapies.


Similar content being viewed by others
Change history
07 November 2017
The authors of the article would like to note an error in the acknowledgements section of this paper.
07 November 2017
The authors of the article would like to note an error in the acknowledgements section of this paper.
07 November 2017
The authors of the article would like to note an error in the acknowledgements section of this paper.
References
Santos F, Fuente R, Mejia N, Mantecon L, Gil-Peña H, Ordoñez FA. Hypophosphatemia and growth. Pediatric Nephrology. 2013;28:595–603.
Wagner CA, Rubio-Aliaga I, Biber J, Hernando N. Genetic diseases of renal phosphate handling. Nephrol Dial Transplant England. 2014;29:45–54.
Biber J, Stieger B, Stange G, Murer H. Isolation of renal proximal tubular brush-border membranes. Nature Protocols England. 2007;2:1356–9.
Marks J, Debnam ES, Unwin RJ. Phosphate homeostasis and the renal-gastrointestinal axis. American Journal of Physiology. Renal Physiology. 2010;299:285–96.
Villa-Bellosta R, Ravera S, Sorribas V, Stange G, Levi M, Murer H, Biber J, Forster IC. The Na + −pi cotransporter PiT-2 (SLC20A2) is expressed in the apical membrane of rat renal proximal tubules and regulated by dietary pi. American Journal of Physiology. Renal Physiology. 2009;296:691–9.
Forster IC, Hernando N, Biber J, Murer H. Proximal tubular handling of phosphate: a molecular perspective. Kidney International. 2006;70:1548–59.
Brenza HL, Kimmel-Jehan C, Jehan F, Shinki T, Wakino S, Anazawa H, Suda T, DeLuca HF. Parathyroid hormone activation of the 25-hydroxyvitamin D3-1alpha-hydroxylase gene promoter. Proceedings of the National Academy of Sciences. 1998;95:1387–91.
Kogawa M, Findlay DM, Anderson PH, Ormsby R, Vincent C, Morris HA, Atkins GJ. Osteoclastic metabolism of 25(OH)-vitamin D3: a potential mechanism for optimization of bone resorption. Endocrinology. 2010;151:4613–25.
Liu S, Zhou J, Tang W, Menard R, Feng JQ, Quarles LD. Pathogenic role of Fgf23 in Dmp1-null mice. American Journal of Physiology. Endocrinology and Metabolism. 2008;295:E254–61.
Ubaidus S, Li M, Sultana S, de Freitas PHL, Oda K, Maeda T, Takagi R, Amizuka N. FGF23 is mainly synthesized by osteocytes in the regularly distributed osteocytic lacunar canalicular system established after physiological bone remodeling. Journal of Electron Microscopy. 2009;58:381–92.
Quarles LD. Skeletal secretion of FGF-23 regulates phosphate and vitamin D metabolism. Nature Reviews. Endocrinology. 2012;8:276–86.
Larsson T, Marsell R, Schipani E, Ohlsson C, Ljunggren O, Tenenhouse HS, Jüppner H, Jonsson KB. Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology. 2004;145:3087–94.
Martin A, David V, Quarles LD. Regulation and Function of the FGF23/Klotho Endocrine Pathways. Physiological Reviews. 2012;92:131–55.
Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–4.
Toro CL. Rol de Klotho y FGF23 en la regulación de fosfato y calcio plasmático. Rev Hosp Clin Univ Chile. 2010;23:25–32.
Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M. Regulation of fibroblast growth factor-23 signaling by klotho. The Journal of Biological Chemistry. 2006;281:6120–3.
Farrow EG, Summers LJ, Schiavi SC, McCormick JA, Ellison DH, White KE. Altered renal FGF23-mediated activity involving MAPK and Wnt: effects of the hyp mutation. The Journal of Endocrinology. 2010;207:67–75.
Ornitz DM, Itoh N. The fibroblast growth factor signaling pathway. Review of Development Biology. 2015;4:215–66.
Sopjani M, Rinnerthaler M, Almilaji A, Ahmeti S, Dermaku-Sopjani M. Regulation of cellular transport by klotho protein. Current Protein & Peptide Science. 2014;15:828–35.
Razzaque MS. The FGF23-klotho axis: endocrine regulation of phosphate homeostasis. Nature Reviews. Endocrinology. 2009;5:611–9.
Webster R, Sheriff S, Faroqui R, Siddiqui F, Hawse JR, Amlal H. Klotho/fibroblast growth factor 23- and PTH-independent estrogen receptor-alpha-mediated direct downregulation of NaPi-IIa by estrogen in the mouse kidney. American Journal of Physiology. Renal Physiology. 2016;311:F249–59.
Dermaku-Sopjani M, Sopjani M, Saxena A, Shojaiefard M, Bogatikov E, Alesutan I, Eichenmüller M, Lang F. Downregulation of NaPi-IIa and NaPi-IIb Na-coupled phosphate transporters by coexpression of klotho. Cellular Physiology and Biochemistry. 2011;28:251–8.
Imura A, Tsuji Y, Murata M, Maeda R, Kubota K, Iwano A, Obuse C, Togashi K, Tominaga M, Kita N, Tomiyama K, Iijima J, Nabeshima Y, Fujioka M, Asato R, Tanaka S, Kojima K, Ito J, Nozaki K, Hashimoto N, Ito T, Nishio T, Uchiyama T, Fujimori T, Nabeshima Y. Alpha-klotho as a regulator of calcium homeostasis. Science. 2007;316:1615–8.
Chang Q, Hoefs S, Van Der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG. The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–3.
Andrukhova O, Smorodchenko A, Egerbacher M, Streicher C, Zeitz U, Goetz R, Shalhoub V, Mohammadi M, Pohl EE, Lanske B, Erben RG. FGF23 promotes renal calcium reabsorption through the TRPV5 channel. The EMBO Journal. 2014;33(3):229–46.
Burnett CH, Dent CE, Harper C, Warland BJ. Vitamin d-resistant rickets. Analysis of twenty-four pedigrees with hereditary and sporadic cases. The American Journal of Medicine. 1964;36:222–32.
Ramussen H, Anast C. Familial hypophosphatemic rickets and vitamin D-dependent rickets. In: Wyngaarden JB, Fredericson DS, Goldstain JL, Brrown MS, editors. The Metabolic Basis of Inherit Disease. N Y: McGraw-Hill; 1983. p. 1743–73.
Collins JF, Bulus N, Ghishan FK. Sodium-phosphate transporter adaptation to dietary phosphate deprivation in normal and hypophosphatemic mice. American Journal of Physics. 1995;268:917–24.
Eicher EM, Southard JL, Scriver CR, Glorieux FH. Hypophosphatemia - mouse model for human familial hypophosphatemic (vitamin-D-resistant) rickets. Proceedings of the National Academy of Sciences. 1976;73:4667–71.
Strom TM, Francis F, Lorenz B, Böddrich A, Econs MJ, Lehrach H, Meitinger T. Pex gene deletions in Gy and hyp mice provide mouse models for X-linked hypophosphatemia. Human Molecular Genetics. 1997;6:165–71.
Tenenhouse H. X-linked hypophosphataemia: a homologous disorder in humans and mice. Nephrology, Dialysis, Transplantation. 1999;333–41
Tenenhouse HS, Beck L. Renal Na + −phosphate cotransporter gene expression in X-linked hyp and Gy mice. Kidney International. 1996;49:1027–32.
Razali NN, Hwu TT, Thilakavathy K. Phosphate homeostasis and genetic mutations of familial hypophosphatemic rickets. Journal of Pediatric Endocrinology & Metabolism. 2015;28:1009–17.
The HYP Consortium. A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nature Genetics. 1995;11:130–6.
Dixon PH, Christie PT, Wooding C, Trump D, Grieff M, Holm I, Gertner JM, Schmidtke J, Shah B, Shaw N, Smith C, Tau C, Schlessinger D, Whyte MP, Thakker RV. Mutational analysis of PHEX Gene in X-liked hypophosphatemia. The Journal of Clinical Endocrinology and Metabolism. 2014;83:3615–23.
Beck L, Soumounou Y, Martel J, Krishnamurthy G, Gauthier C, Goodyer CG, Tenenhouse HS. Pex/PEX tissue distribution and evidence for a deletion in the 3′ region of the Pex gene in X-linked hypophosphatemic mice. The Journal of Clinical Investigation. 1997;99:1200.
Bastepe M, Juppner H. Inherited hypophosphatemic disorders in children and the evolving mechanisms of phosphate regulation. Reviews in Endocrine & Metabolic Disorders. 2008;9:171–80.
Meyer RAJ, Tenenhouse HS, Meyer MH, Klugerman AH. The renal phosphate transport defect in normal mice parabiosed to X-linked hypophosphatemic mice persists after parathyroidectomy. Journal of Bone and Mineral Research. 1989;4:523–32.
Tenenhouse HS, Martel J, Gauthier C, Segawa H, Miyamoto K. Differential effects of Npt2a gene ablation and X-linked hyp mutation on renal expression of Npt2c. American Journal of Physiology. Renal Physiology. 2003;285:1271–8.
Liu S, Tang W, Zhou J, Vierthaler L, Quarles LD. Distinct roles for intrinsic osteocyte abnormalities and systemic factors in regulation of FGF23 and bone mineralization in hyp mice. American Journal of Physiology. Endocrinology and Metabolism. 2007;293:1636–44.
Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, Takeuchi Y, Fujita T, Nakahara K, Yamashita T, Fukumoto S. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. The Journal of Clinical Endocrinology and Metabolism. 2002;87:4957–60.
Chanakul A, Zhang MY, Louw A, Armbrecht HJ, Miller WL, Portale AA, Perwad F. FGF-23 regulates CYP27B1 transcription in the kidney and in extra-renal tissues. PLoS One. 2013;8:72816.
Reid IR, Murphy WA, Hardy DC, Teitelbaum SL, Bergfeld MA, Whyte MP. X-linked hypophosphatemia: skeletal mass in adults assessed by histomorphometry, computed tomography, and absorptiometry. The American Journal of Medicine. 1991;90:63–9.
Klaus SSB, Lars B. Phenotype presentation of hypophosphatemic rickets in adults. Calcified Tissue International. 2010;87:108–19.
Weber TJ, Liu S, Indridason OS, Quarles LD. Serum FGF23 levels in normal and disordered phosphorus homeostasis. Journal of Bone and Mineral Research. 2003;18:1227–34.
Schutt SM, Schumacher M, Holterhus PM, Felgenhauer S, Hiort O. Effect of GH replacement therapy in two male siblings with combined X-linked hypophosphatemia and partial GH deficiency. European Journal of Endocrinology. 2003;149:317–21.
Mäkitie O, Doria A, Kooh SW, Cole WG, Daneman A, Sochett E. Early treatment improves growth and biochemical and radiographic outcome in X-linked hypophosphatemic rickets. The Journal of Clinical Endocrinology and Metabolism. 2003;88:3591–7.
Baroncelli GI, Bertelloni S, Ceccarelli C, Saggese G. Effect of growth hormone treatment on final height, phosphate metabolism, and bone mineral density in children with X-linked hypophosphatemic rickets. The Journal of Pediatrics. 2001;138:236–43.
Whyte MP, Schranck FW, Armamento-Villareal R. X-linked hypophosphatemia: a search for gender, race, anticipation, or parent of origin effects on disease expression in children. The Journal of Clinical Endocrinology and Metabolism. 1996;81:4075–80.
Živičnjak M, Schnabel D, Billing H, Staude H, Filler G, Querfeld U, Schumacher M, Pyper A, Schröder C, Brämswig J, Haffner D. Hypophosphatemic Rickets Study Group of Arbeitsgemeinschaft für Pädiatrische Endokrinologie and Gesellschaft für Pädiatrische Nephrologie. Age-related stature and linear body segments in children with X-linked hypophosphatemic rickets. Pediatric Nephrology. 2011;26:223–31.
Linglart A, Biosse-Duplan M, Briot K, Chaussain C, Esterle L, Guillaume-Czitrom S, Kamenicky P, Nevoux J, Prié D, Rothenbuhler A, Wicart P, Harvengt P. Therapeutic management of hypophosphatemic rickets from infancy to adulthood. Endocrine Connections. 2014;3:13–30.
Jehan F, Gaucher C, Nguyen TM, Walrant-Debray O, Lahlou N, Sinding C, Déchaux M, Garabédian M. Vitamin D receptor genotype in hypophosphatemic rickets as a predictor of growth and response to treatment. The Journal of Clinical Endocrinology and Metabolism. 2008;93:4672–82.
McNair, Stickler. Growth in familial hypophosphatemic vitamin-D-resistant rickets. NEJM. 1969;281:511–6.
Oliveri MB, Cassinelli H, Bergada C, Mautalen CA. Bone mineral density of the spine and radius shaft in children with X-linked hypophosphatemic rickets (XLH). Bone and Mineral. 1991;12:91–100.
Friedman NE, Lobaugh B, Drezner MK. Effects of calcitriol and phosphorus therapy on the growth of patients with X-linked hypophosphatemia. The Journal of Clinical Endocrinology and Metabolism. 1993;76:839–44.
Qiu ZQ, Travers R, Rauch F, Glorieux FH, Scriver CR, Tenenhouse HS. Effect of gene dose and parental origin on bone histomorphometry in X-linked hyp mice. Bone. 2004;34:134–9.
Hunziker EB. Mechanism of longitudinal bone growth and its regulation by growth plate chondrocytes. Microscopy Research and Technique. 1994;28:505–19.
Liu S, Guo R, Quarles LD. Cloning and characterization of the proximal murine Phex promoter. Endocrinology. 2001;142:3987–95.
Miao D, Bai X, Panda D, McKee M, Karaplis A, Goltzman D. Osteomalacia in hyp mice is associated with abnormal Phex expression and with altered bone matrix protein expression and deposition. Endocrinology. 2001;142:926–39.
Miao D, Bai X, Panda DK, Karaplis AC, Goltzman D, McKee MD. Cartilage abnormalities are associated with abnormal Phex expression and with altered matrix protein and MMP-9 localization in hyp mice. Bone. 2004;34:638–47.
Wu S, Levenson A, Kharitonenkov A, De Luca F. Fibroblast growth factor 21 (FGF21) inhibits chondrocyte function and growth hormone action directly at the growth plate. The Journal of Biological Chemistry. 2012;287:26060–7.
Gunther T, Chen Z-F, Kim J, Priemel M, Rueger JM, Amling M, Moseley JM, Martin TJ, Anderson DJ, Karsenty G. Genetic ablation of parathyroid glands reveals another source of parathyroid hormone. Nature. 2000;406:199–203.
Tu Q, Pi M, Karsenty G, Simpson L, Liu S, Quarles LD. Rescue of the skeletal phenotype in CasR-deficient mice by transfer onto the Gcm2 null background. The Journal of Clinical Investigation. 2003;111:1029–37.
Sabbagh Y, Carpenter TO, Demay MB. Hypophosphatemia leads to rickets by impairing caspase-mediated apoptosis of hypertrophic chondrocytes. Proceedings of the National Academy of Sciences. 2005;102:9637–42.
Liu S, Zhou J, Tang W, Jiang X, Rowe DW, Quarles LD. Pathogenic role of Fgf23 in hyp mice. American Journal of Physiology. Endocrinology and Metabolism. 2006;291:38–49.
Ovejero D, Lim YH, Boyce AM, Gafni RI, McCarthy E, Nguyen TA, Eichenfield LF, DeKlotz CM, Guthrie LC, Tosi LL, Thornton PS, Choate KA, Collins MT. Cutaneous skeletal hypophosphatemia syndrome: clinical spectrum, natural history, and treatment. Osteoporosis International. 2016;27:3615–26.
Goodyer PR, Kronick JB, Jequier S, Reade TM, Scriver CR. Nephrocalcinosis and its relationship to treatment of hereditary rickets. The Journal of Pediatrics. 1987;111:700–4.
Sanchez CP. Mineral metabolism and bone abnormalities in children with chronic renal failure. Reviews in Endocrine & Metabolic Disorders. 2008;9:131–7.
Imel EA, DiMeglio LA, Hui SL, Carpenter TO, Econs MJ. Treatment of X-linked hypophosphatemia with calcitriol and phosphate increases circulating fibroblast growth factor 23 concentrations. The Journal of Clinical Endocrinology and Metabolism. 2010;95:1846–50.
Nickolas TL, Jamal SA. Bone kidney interactions. Reviews in Endocrine & Metabolic Disorders. 2015;16:157–63.
Wilson DM, Lee PD, Morris AH, Reiter EO, Gertner JM, Marcus R, Valerie E. Ron G. Growth hormone therapy in hypophosphatemic rickets. American Journal of Diseases of Children (1911). 1991;145:1165–70.
Cameron FJ, Sochett EB, Daneman A, Kooh SW. A trial of growth hormone therapy in well-controlled hypophosphataemic rickets. Clinical Endocrinology. 1999;50:577–82.
Haffner D, Nissel R, Wuhl E, Mehls O. Effects of growth hormone treatment on body proportions and final height among small children with X-linked hypophosphatemic rickets. Pediatrics. 2004;113:593–6.
Roy P, Martel J, Tenenhouse H. Growth hormone normalizes renal 1,25-dihydroxyvitamin D3-24-hydroxylase gene expression but not Na + −phosphate cotransporter (Npt2) mRNA in phosphate-deprived hyp mice. Journal of Bone and Mineral Research. 1997;12:1672–80.
Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6500–5.
White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney International. 2001;60:2079–86.
Wöhrle S, Bonny O, Beluch N, Gaulis S, Stamm C, Scheibler M, Müller M, Kinzel B, Thuery A, Brueggen J, Hynes NE, Sellers WR, Hofmann F, Graus-Porta D. FGF receptors control vitamin D and phosphate homeostasis by mediating renal FGF-23 signaling and regulating FGF-23 expression in bone. Journal of Bone and Mineral Research. 2011;26:2486–97.
Li H, Martin A, David V, Quarles LD. Compound deletion of Fgfr3 and Fgfr4 partially rescues the hyp mouse phenotype. American Journal of Physiology. Endocrinology and Metabolism. 2011;300:508–17.
Bai X, Miao D, Xiao S, Qiu D, St-Arnaud R, Petkovich M, Gupta A, Goltzman D, Karaplis AC. CYP24 inhibition as a therapeutic target in FGF23-mediated renal phosphate wasting disorders. The Journal of Clinical Investigation. 2016;126:667–80.
Wöhrle S, Henninger C, Bonny O, Thuery A, Beluch N, Hynes NE, Guagnano V, Sellers WR, Hofmann F, Kneissel M, Graus PD. Pharmacological inhibition of fibroblast growth factor (FGF) receptor signaling ameliorates FGF23-mediated hypophosphatemic rickets. Journal of Bone and Mineral Research. 2013;28:899–911.
Du E, Xiao L, Hurley MM. FGF23 neutralizing antibody ameliorates hypophosphatemia and impaired FGF receptor signaling in kidneys of HMWFGF2 transgenic mice. Journal of Cellular Physiology. 2016; doi:10.1002/jcp.25458.
Aono Y, Yamazaki Y, Yasutake J, Kawata T, Hasegawa H, Urakawa I, Fujita T, Wada M, Yamashita T, Fukumoto S, Shimada T. Therapeutic effects of anti-FGF23 antibodies in hypophosphatemic rickets/osteomalacia. Journal of Bone and Mineral Research. 2009;24:1879–88.
Ranch D, Zhang MYH, Portale AA, Perwad F. Fibroblast growth factor 23 regulates renal 1,25-dihydroxyvitamin D and phosphate metabolism via the MAP kinase signaling pathway in hyp mice. Journal of Bone and Mineral Research. 2011;26:1883–90.
Zhang MYH, Ranch D, Pereira RC, Armbrecht HJ, Portale AA, Perwad F. Chronic inhibition of ERK1/2 signaling improves disordered bone and mineral metabolism in hypophosphatemic (hyp) mice. Endocrinology. 2012;153:1806–16.
Imel EA, Zhang X, Ruppe MD, Weber TJ, Klausner MA, Ito T, Vergeire M, Humphrey JS, Glorieux FH, Portale AA, Insogna K, Peacock M, Carpenter TO. Prolonged correction of serum phosphorus in adults with X-linked hypophosphatemia using monthly doses of KRN23. The Journal of Clinical Endocrinology and Metabolism. 2015;100:2565–73.
Carpenter TO, Imel EA, Ruppe MD, Weber TJ, Klausner MA, Wooddell MM, Kawakami T, Ito T, Zhang X, Humphrey J, Insogna KL, Peacock M. Randomized trial of the anti-FGF23 antibody KRN23 in X-linked hypophosphatemia. The Journal of Clinical Investigation. 2014;124:1587–97.
Acknowledgements
This work was supported by the National Plan I + D + I 2008-2011, Instituto de Salud Carlos III (PI12/00987) and also by the National Plan I + D + I 2013-2016 Instituto de Salud Carlos III (PI14/00702 and PI15/02122), European Regional Development Funds 2013-2016 (ERDF, Grupín 14-020) and by the Foundation of the University of Oviedo (FUO).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The article did not involve human or animal participation. Therefore, informed consent or IRB approval were not needed.
Conflict of interest
The authors declare that they have no conflict of interest.
•The manuscript has not been submitted to more than one journal.
•The manuscript has not been published previously.
•No data/figures have been fabricated or manipulated.
•A single study is not split up into several parts.
•No data, text or theories are being plagiarized.
•All authors have been informed about the submission of the paper and all have contributed to the article.
Rights and permissions
About this article
Cite this article
Fuente, R., Gil-Peña, H., Claramunt-Taberner, D. et al. X-linked hypophosphatemia and growth. Rev Endocr Metab Disord 18, 107–115 (2017). https://doi.org/10.1007/s11154-017-9408-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-017-9408-1