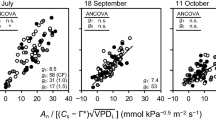
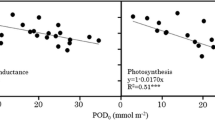
Abstract
Industrialization has significantly altered atmospheric chemistry by increasing concentrations of chemicals such as nitrogen oxides (NO x ) and volatile organic carbon, which react in the presence of sunlight to produce tropospheric ozone (O3). Ozone is a powerful oxidant that causes both visual and physiological damage to plants, impairing the ability of the plant to control processes like photosynthesis and transpiration. Damage to photosynthesis and stomatal conductance does not always occur at the same rate, which generates a problem when using the Ball-Berry model to predict stomatal conductance because the calculations directly rely on photosynthesis rates. The goals of this work were to develop a modeling framework to modify Ball-Berry stomatal conductance predictions independently of photosynthesis and to test the framework using experimental data. After exposure to elevated O3 in open-top chambers, photosynthesis and stomatal conductance in tulip poplar changed at different rates through time. We were able to accurately model observed photosynthetic and stomatal conductance responses to chronic O3 exposure in a Ball-Berry framework by adjusting stomatal conductance in addition to photosynthesis. This led to a significant improvement in the modeled ability to predict both photosynthesis and stomatal conductance responses to O3.




Similar content being viewed by others
References
Ball JT, Woodrow IE, JA Berry (ed) (1987) A model predicting stomatal conductance and its contribution to the control of photosynthesis under different environmental conditions. In: Biggens J (ed) Progress in photosynthesis research. Martinus Nijhoff, The Hague, pp 221–224
Bonan GB (2008) Forests and climate change: forcings, feedbacks, and the climate benefits of forests. Science 320:1444–1449
Bonan GB et al (2011) Improving canopy processes in the community land model version 4 (clm4) using global flux fields empirically inferred from fluxnet data. J Geophys Res Biogeosci 116:22
Bortier K, Ceulemans R, De Temmerman L (2000) Effects of ozone exposure on growth and photosynthesis of beach seedlings (Fagus sylvatica). New Phytol 146:271–280
Collatz GJ, Ball JT, Grivet C, Berry JA (1991) Physiological and environmental-regulation of stomatal conductance, photosynthesis and transpiration—a model that includes a laminar boundary-layer. Agric For Meteorol 54:107–136
Constable J, Taylor G (1997) Modeling the effects of elevated tropospheric O3 on two varieties of Pinus ponderosa. Can J For Res 27:527–537
Cowan I (1978) Stomatal behaviour and environment. Adv Bot Res 4:117–228
de Beeck MO, Loew M, Verbeeck H, Deckmyn G (2007) Suitability of a combined stomatal conductance and photosynthesis model for calculation of leaf-level ozone fluxes. Plant Biol 9:331–341
Deckmyn G et al (2007) Modelling ozone effects on adult beech trees through simulation of defence, damage, and repair costs: implementation of the CASIROZ ozone model in the ANAFORE forest model. Plant Biol 9:320–330
Farage PK, Long SP (1999) The effects of O3 fumigation during leaf development on photosynthesis of wheat and pea: an in vivo analysis. Photosynth Res 59:1–7
Farage P, Long S, Lechner E, Baker N (1991) The sequence of change within the photosynthetic apparatus of wheat following short-term exposure to ozone. Plant Physiol 95:529–535
Farquhar G, Caemmerer S, Berry J (1980) A biochemical-model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90
Felzer B, Kicklighter D, Melillo J, Wang C, Zhuang Q, Prinn R (2004) Effects of ozone on net primary production and carbon sequestration in the conterminous United States using a biogeochemistry model. Tellus B 56:230–248
Feng Z, Kobayashi K, Ainsworth EA (2008) Impact of elevated ozone concentration on growth, physiology and yield of wheat (Triticum aestivum L.): a meta-analysis. Global Change Biol 14:2696–2708
Fiscus E, Reid C, Miller J, Heagle A (1997) Elevated CO2 reduces O3 flux and O3-induced yield losses in soybeans: possible implications for elevated CO2 studies. J Exp Bot 48:307–313
Fiscus E, Booker F, Burkey K (2005) Crop responses to ozone: uptake, modes of action, carbon assimilation and partitioning. Plant Cell Environ 28:997–1011
Francini A, Nali C, Picchi V, Lorenzini G (2007) Metabolic changes in white clover clones exposed to ozone. Environ Exp Bot 60:11–19
Freersmith PH, Dobson MC (1989) Ozone flux to Picea sitchensis (Bong) carr and Picea abies (L.) karst during short episodes and the effects of these on transpiration and photosynthesis. Environ Pollut 59:161–176
Harley P, Sharkey T (1991) An improved model of C3 photosynthesis at high CO2-reversed O2 sensitivity explained by lack of glycerate reentry into the chloroplast. Photosynth Res 27:169–178
Harley P, Thomas R, Reynolds J, Strain B (1992) Modeling photosynthesis of cotton grown in elevated CO2. Plant Cell Environ 15:271–282
Hassan IA, Ashmore MR, Bell JNB (1994) Effects of O3 on the stomatal behavior of Egyptian varieties of radish (Raphanus sativus L. cv Baladey) and turnip (Brassica rapa L. cv Sultani). New Phytol 128:243–249
Heagle AS, Reinert RA, Miller JE (1996) Response of white clover to ozone in different environments. J Environ Qual 25:273–278
Kellomaki S, Wang KY (1997) Effects of elevated O3 and CO2 concentrations on photosynthesis and stomatal conductance in scots pine. Plant Cell Environ 20:995–1006
Maiermaercker U, Koch W (1991) Experiments on the control capacity of stomata of Picea abies (L.) Karst after fumigation with ozone and in environmentally damaged material. Plant Cell Environ 14:175–184
Manes F, Donato E, Vitale M (2001) Physiological response of Pinus halepensis needles under ozone and water stress conditions. Physiol Plant 113:249–257
Martin M, Farage P, Humphries S, Long S (2000) Can the stomatal changes caused by acute ozone exposure be predicted by changes occurring in the mesophyll? A simplification for models of vegetation response to the global increase in tropospheric elevated ozone episodes. Aust J Plant Physiol 27:211–219
McLaughlin SB, Wullschleger SD, Sun G, Nosal M (2007) Interactive effects of ozone and climate on water use, soil moisture content and streamflow in a southern Appalachian forest in the USA. New Phytol 174:125–136
Mills G, Hayes F, Wilkinson S (2009) Chronic exposure to increasing background ozone impairs stomatal functioning in grassland species. Glob Change Biol 15:1522–1533
Morgan PB, Bernacchi CJ, Ort DR, Long SP (2004) An in vivo analysis of the effect of season-long open-air elevation of ozone to anticipated 2050 levels on photosynthesis in soybean. Plant Physiol 135:2348–2357
Noormets A et al (2001) Stomatal and non-stomatal limitation to photosynthesis in two trembling aspen (Populus tremuloides Michx.) clones exposed to elevated CO2 and/or O3. Plant Cell Environ 24:327–336
Nunn AJ et al (2006) Testing the unifying theory of ozone sensitivity with mature trees of Fagus sylvatica and Picea abies. Tree Physiol 26:1391–1403
Ojanpera K, Patsikka E, Ylaranta T (1998) Effects of low ozone exposure of spring wheat on net CO2 uptake, Rubisco, leaf senescence and grain filling. New Phytol 138:451
Ollinger S, Aber J, Reich P (1997) Simulating ozone effects on forest productivity: interactions among leaf-, canopy-, and stand-level processes. Ecol Appl 7:1237–1251
Panek JA (2004) Ozone uptake, water loss and carbon exchange dynamics in annually drought-stressed Pinus ponderosa forests: measured trends and parameters for uptake modeling. Tree Physiol 24:277–290
Paoletti E (2005) Ozone slows stomatal response to light and leaf wounding in a Mediterranean evergreen broadleaf, Arbutus unedo. Environ Pollut 134:439–445
Paoletti E, Grulke NE (2005) Does living in elevated CO2 ameliorate tree response to ozone? A review on stomatal responses. Environ Pollut 137:483–493
Paoletti E, Grulke NE (2010) Ozone exposure and stomatal sluggishness in different plant physiognomic classes. Environ Pollut 158:2664–2671
Paoletti E, Nali C, Lorenzini G (2007) Early responses to acute ozone exposure in two Fagus sylvatica clones differing in xeromorphic adaptations: photosynthetic and stomatal processes, membrane and epicuticular characteristics. Environ Monit Assess 128:93–108
Reich PB (1987) Quantifying plant response to ozone: a unifying theory. Tree Physiol 3:63–91
Reichenauer T, BolharNordenkampf HR, Ehrlich U, Soja G, Postl WF, Halbwachs F (1997) The influence of ambient and elevated ozone concentrations on photosynthesis in Populus nigra. Plant Cell Environ 20:1061–1069
Sharkey TD, Bernacchi CJ, Farquhar GD, Singsaas EL (2007) Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ 30:1035–1040
Sharma P, Sober A, Sober J, Podila GK, Kubiske ME, Mattson WJ, Isebrands JG, Karnosky DF (2003) Moderation of [CO2]-induced gas exchange responses by elevated tropospheric O3 in trembling aspen and sugar maple. Ekologia (Bratislava) 22:318–331
Sitch S, Cox PM, Collins WJ, Huntingford C (2007) Indirect radiative forcing of climate change through ozone effects on the land-carbon sink. Nature 448:791–794
Skelly JM et al (1999) Observation and confirmation of foliar ozone symptoms of native plant species of Switzerland and southern Spain. Water Air Soil Pollut 116:227–234
Tjoelker MG, Volin JC, Oleksyn J, Reich PB (1995) Interaction of ozone pollution and light effects on photosynthesis in a forest canopy experiment. Plant Cell Environ 18:895–905
Torsethaugen G, Pell E, Assmann S (1999) Ozone inhibits guard cell K+ channels implicated in stomatal opening. Proc Natl Acad Sci USA 96:13577–13582
von Caemmerer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas-exchange of leaves. Planta 153:376–387
Wittig VE, Ainsworth EA, Long SP (2007) To what extent do current and projected increases in surface ozone affect photosynthesis and stomatal conductance of trees? A meta-analytic review of the last 3 decades of experiments. Plant Cell Environ 30:1150–1162
Zhang L, Xu H, Yang J-C, Li W-D, Jiang G-M, Li Y-G (2010) Photosynthetic characteristics of diploid honeysuckle (Lonicera japonica thunb.) and its autotetraploid cultivar subjected to elevated ozone exposure. Photosynthetica 48:87–95
Acknowledgments
The Cornell NSF Interdisciplinary Graduate Education, Research, and Training (IGERT) in Biogeochemistry and Environmental Biocomplexity provided funding for this study. Many thanks to Allyson Eller, Carrie McCalley and Dena Vallano for help with setting up and monitoring the fumigation system, and to Natalie Mahowald, Peter Hess, and Christine Goodale for helpful comments and insights in preparing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Robert Pearcy.
Rights and permissions
About this article
Cite this article
Lombardozzi, D., Sparks, J.P., Bonan, G. et al. Ozone exposure causes a decoupling of conductance and photosynthesis: implications for the Ball-Berry stomatal conductance model. Oecologia 169, 651–659 (2012). https://doi.org/10.1007/s00442-011-2242-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-011-2242-3