Abstract
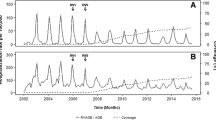
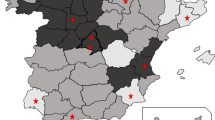
The objective of this study was to estimate, by a novel spatiotemporal approach in an environment of non-funded rotavirus (RV) vaccines, the RV vaccine effectiveness (VE) to prevent acute gastroenteritis primary care (AGE-PC)–attended episodes, demonstrating how indirect protection leads to underestimation of direct VE under high vaccine coverage (VC). This population-based retrospective cohort study used electronic healthcare registries including all children 2 months–5 years old, born from 2009 to 2018 in the Valencia Region (Spain). Direct RV VE preventing AGE-PC episodes was estimated using propensity score matching and Poisson regressions stratified by VC, adjusted by age and calendar season. Indirect VE was estimated by Poisson regression comparing AGE-PC rates in unvaccinated children among the different VC levels. A total of 563,442 children were included for the RV VC estimation; of them, 360,576 were included in the birth-cohort for VE analysis. RV VC showed strong variability among districts and seasons, rising on average from 21% in 2009/2010 to 55% in 2017/2018. The highest direct VE was found in vaccinated children from districts with 0–30% RV VC (16.4%) and the lowest in those from districts with ≥ 70% RV VC (9.7%). The indirect protection in unvaccinated children raised from 6 to 16.6% for those living with 20–30% and ≥ 70% VC, respectively.
Conclusion: Considering that RV is the causative agent in 20% of AGE cases, a direct effectiveness of 82% preventing AGE-PC episodes due to RV could be deduced using a novel spatiotemporal approach. A reduction of 17% of AGE-PC episodes in unvaccinated was observed in areas with VC over 70% because of indirect protection.
What is Known: • The effectiveness of RV vaccines preventing hospitalizations due to RV-acute gastroenteritis (RV-AGE) has been extensively studied. However, RV also burdens the primary care (PC) setting, and data on vaccine effectiveness (VE) in preventing AGE-PC visits are scarce. • The RV vaccine distribution in Spain (non-funded), with large differences in vaccine coverage (VC) among healthcare districts, provides an ideal scenario to assess the actual VE in preventing AGE-PC consultations, including the direct and indirect protection. | |
What is New: • A direct effectiveness of 82% preventing AGE-PC episodes due to RV could be deduced using a novel spatiotemporal approach. A reduction of 17% of AGE-PC episodes in unvaccinated was observed in areas with high VC because of indirect protection. • These findings, together with existing data on the impact on hospitalizations due to RV-AGE, offer valuable insights for implementing vaccination initiatives in countries that have not yet commenced such programs. |


Similar content being viewed by others
Data availability
Authors agree to make data and materials supporting the results presented in the paper available upon reasonable request.
Abbreviations
- AGE:
-
Acute gastroenteritis
- AGE-PC:
-
Acute gastroenteritis primary care
- HCSB:
-
Healthcare seeking behaviour
- ICD-9-MC:
-
55International Classification of Diseases, Ninth Revision, Clinical Modification coding system
- ICD-10-MC:
-
International Classification of Diseases, Ninth Revision, Clinical Modification coding system
- PC:
-
Primary care
- PSM:
-
Propensity score matching
- RV:
-
Rotavirus
- RVAGE:
-
Gastroenteritis due to RV
- SMD:
-
Standardized mean differences
- VC:
-
Vaccine coverage
- VE:
-
Vaccine effectiveness
- VID:
-
The Valencia healthcare Integrated Databases
- VIS:
-
Vaccine Information System
References
Troeger C, Khalil IA, Rao PC, Cao S, Blacker BF, Ahmed T et al (2018) Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr 172:958–965. https://doi.org/10.1001/jamapediatrics.2018.1960
López-Lacort M, Orrico-Sánchez A, Martínez-Beneito MÁ, Muñoz-Quiles C, Díez-Domingo J (2020) Spatio-temporal impact of self-financed rotavirus vaccination on rotavirus and acute gastroenteritis hospitalisations in the Valencia region. Spain BMC Infect Dis 20:656. https://doi.org/10.1186/s12879-020-05373-0
Orrico-Sanchez A, López-Lacort M, Pérez-Vilar S, Díez-Domingo J (2017) Long-term impact of self-financed rotavirus vaccines on rotavirus-associated hospitalizations and costs in the Valencia Region. Spain BMC Infect Dis 17:267. https://doi.org/10.1186/s12879-017-2380-2
Clark A, van Zandvoort K, Flasche S, Sanderson C, Bines J, Tate J et al (2019) Efficacy of live oral rotavirus vaccines by duration of follow-up: a meta-regression of randomised controlled trials. Lancet Infect Dis 19:717–727. https://doi.org/10.1016/S1473-3099(19)30126-4
Payne DC, Selvarangan R, Azimi PH, Boom JA, Englund JA, Staat MA et al (2015) Long-term consistency in rotavirus vaccine protection: RV5 and RV1 vaccine effectiveness in US children, 2012–2013. Clin Infect Dis Off Publ Infect Dis Soc Am 61:1792–1799. https://doi.org/10.1093/cid/civ872
Rosettie KL, Vos T, Mokdad AH, Flaxman AD, Khalil I, Troeger C et al (2018) Indirect rotavirus vaccine effectiveness for the prevention of rotavirus hospitalization: a systematic review and meta-analysis. Am J Trop Med Hyg 98:1197–1201. https://doi.org/10.4269/ajtmh.17-0705
Panozzo CA, Becker-Dreps S, Pate V, Weber DJ, Jonsson Funk M, Stürmer T et al (2014) Direct, indirect, total, and overall effectiveness of the rotavirus vaccines for the prevention of gastroenteritis hospitalizations in privately insured US children, 2007–2010. Am J Epidemiol 179:895–909. https://doi.org/10.1093/aje/kwu001
Ito M, Higashigawa M (2021) Effectiveness of self-financed rotavirus vaccination in Ise City. Japan Hum Vaccines Immunother 17:5650–5655. https://doi.org/10.1080/21645515.2021.1972706
Marquis A, Koch J (2020) Impact of routine rotavirus vaccination in Germany: evaluation five years after its introduction. Pediatr Infect Dis J 39:e109–e116. https://doi.org/10.1097/INF.0000000000002656
Paulke-Korinek M, Kollaritsch H, Aberle SW, Zwazl I, Schmidle-Loss B, Vécsei A et al (2013) Sustained low hospitalization rates after four years of rotavirus mass vaccination in Austria. Vaccine 31:2686–2691. https://doi.org/10.1016/j.vaccine.2013.04.001
Solastie A, Leino T, Ollgren J (2020) Success ofrotavirus vaccination in Finland, a register based study measuring impact beyond overall effectiveness. Vaccine 38:3766–3772. https://doi.org/10.1016/j.vaccine.2020.03.022
Eurostat. Consultatios of a medical doctor in Europe [Internet] https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Healthcare_activities_statistics_-_consultations. Accessed 30 July 2022
Jit M, Bilcke J, Mangen M-JJ, Salo H, Melliez H, Edmunds WJ et al (2009) The cost-effectiveness of rotavirus vaccination: comparative analyses for five European countries and transferability in Europe. Vaccine 27(44):6121–8. https://doi.org/10.1016/j.vaccine.2009.08.030
Ardura-Garcia C, Kreis C, Rakic M, Jaboyedoff M, Mallet MC, Low N et al (2021) Rotavirus disease and health care utilisation among children under 5 years of age in highly developed countries: a systematic review and meta-analysis. Vaccine 39:2917–2928. https://doi.org/10.1016/j.vaccine.2021.04.039
Díez-Domingo J, Martín IO, Sanz AB, López AG, Martínez CC, Boronat CP et al (2006) Rotavirus gastroenteritis among children under five years of age in Valencia. Spain Pediatr Infect Dis J 25:455–457. https://doi.org/10.1097/01.inf.0000217378.30444.21
Martinón-Torres F, Alejandro MB, Collazo LR, Lastres JMS, Díaz SP, Pillado MTS et al (2011) Effectiveness of rotavirus vaccination in Spain. Hum Vaccin 7:757–761. https://doi.org/10.4161/hv.7.7.15576
Hungerford D, Vivancos R, Read JM, Bonnett LJ, Bar-Zeev N, Iturriza-Gómara M et al (2018) Mitigating bias in observational vaccine effectiveness studies using simulated comparator populations: application to rotavirus vaccination in the UK. Vaccine 36:6674–6682. https://doi.org/10.1016/j.vaccine.2018.09.051
Muhsen K, Chodick G, Goren S, Shalev V, Cohen D (2010) The uptake of rotavirus vaccine and its effectiveness in preventing acute gastroenteritis in the community. Vaccine 29:91–94. https://doi.org/10.1016/j.vaccine.2010.10.010
Lahariya C (2016) Vaccine epidemiology: a review. J Fam Med Prim Care 5:7–15. https://doi.org/10.4103/2249-4863.184616
Tseng HF, Sy LS (2018) Use of real-world evidence to evaluate the effectiveness of herpes zoster vaccine. J Infect Dis 218:S63–S67. https://doi.org/10.1093/infdis/jiy263
Henkel L, Sprengholz P, Korn L, Betsch C, Böhm R (2022) The association between vaccination status identification and societal polarization. Nat Hum Behav 7: 231-239. https://doi.org/10.1038/s41562-022-01469-6
Weinberg GA, Szilagyi PG (2010) Vaccine epidemiology: efficacy, effectiveness, and the translational research roadmap. J Infect Dis 201:1607–1610. https://doi.org/10.1086/652404
Chen RT, Orenstein WA (1996) Epidemiologic methods in immunization programs. Epidemiol Rev 18:99–117. https://doi.org/10.1093/oxfordjournals.epirev.a017931
Austin PC (2011) An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res 46:399–424. https://doi.org/10.1080/00273171.2011.568786
Remschmidt C, Wichmann O, Harder T (2015) Frequency and impact of confounding by indication and healthy vaccinee bias in observational studies assessing influenza vaccine effectiveness: a systematic review. BMC Infect Dis 15:429. https://doi.org/10.1186/s12879-015-1154-y
López-Lacort M, Muñoz-Quiles C, Orrico-Sánchez A (2023) Meningococcal group B vaccine (4CMenB) in children. N Engl J Med 388:2109. https://doi.org/10.1056/NEJMc2303518
Weitzen S, Lapane KL, Toledano AY, Hume AL, Mor V (2004) Principles for modeling propensity scores in medical research: a systematic literature review. Pharmacoepidemiol Drug Saf 13:841–853. https://doi.org/10.1002/pds.969
McCaffrey DF, Ridgeway G, Morral AR (2004) Propensity score estimation with boosted regression for evaluating causal effects in observational studies. Psychol Methods 9:403–425. https://doi.org/10.1037/1082-989X.9.4.403
García-Sempere A, Orrico-Sánchez A, Muñoz-Quiles C, Hurtado I, Peiró S, Sanfélix-Gimeno G et al (2020) Data resource profile: the Valencia Health System Integrated Database (VID). Int J Epidemiol 49:740–741e. https://doi.org/10.1093/ije/dyz266
Zhang Z, Kim HJ, Lonjon G, Zhu Y (2019) Balance diagnostics after propensity score matching. Ann Transl Med 7(1):16. https://doi.org/10.21037/atm.2018.12.10
Pérez-Vilar S, Díez-Domingo J, López-Lacort M, Martínez-Úbeda S, Martinez-Beneito MA (2015) Effectiveness of rotavirus vaccines, licensed but not funded, against rotavirus hospitalizations in the Valencia Region. Spain BMC Infect Dis 15:92. https://doi.org/10.1186/s12879-015-0811-5
Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SC et al (2006) Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 354:11–22. https://doi.org/10.1056/NEJMoa052434
Bruun T, Salamanca BV, Bekkevold T, Døllner H, Gibory M, Gilje AM et al (2021) Impact of the rotavirus vaccination program in norway after four years with high coverage. Pediatr Infect Dis J 40:368–374. https://doi.org/10.1097/INF.0000000000003020
Patel MM, Steele D, Gentsch JR, Wecker J, Glass RI, Parashar UD (2011) Real-world impact of rotavirus vaccination. Pediatr Infect Dis J 30:S1-5. https://doi.org/10.1097/INF.0b013e3181fefa1f
Hungerford D, Vivancos R, Read JM, Iturriza-Gόmara M, French N, Cunliffe NA (2018) Rotavirus vaccine impact and socioeconomic deprivation: an interrupted time-series analysis of gastrointestinal disease outcomes across primary and secondary care in the UK. BMC Med 16:10. https://doi.org/10.1186/s12916-017-0989-z
Thomas SL, Walker JL, Fenty J, Atkins KE, Elliot AJ, Hughes HE et al (2017) Impact of the national rotavirus vaccination programme on acute gastroenteritis in England and associated costs averted. Vaccine 35:680–686. https://doi.org/10.1016/j.vaccine.2016.11.057
Pérez-Vilar S, Díez-Domingo J, Gomar-Fayos J, Pastor-Villalba E, Sastre-Cantón M, Puig-Barberà J (2003) Post-licensure passive safety surveillance of rotavirus vaccines: reporting sensitivity for intussusception. An Pediatr Barc Spain 2014(81):77–85. https://doi.org/10.1016/j.anpedi.2013.10.027
Álvarez Aldeán J, Ares Segura S, Díaz González C, Montesdeoca Melián A, García Sánchez R, Boix Alonso H et al (2019) Recommendations for vaccination against ROTAvirus in PREMature newborns (ROTAPREM). An Pediatr 91:205.e1-205.e7. https://doi.org/10.1016/j.anpedi.2019.06.001
Funding
Supported in part by a research grant from Investigator-Initiated Studies Program of Merck Sharp & Dohme LLC. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme LLC.
Author information
Authors and Affiliations
Contributions
AOS is the guarantor of the paper, taking responsibility for the integrity of the work as a whole, from inception to published article.
MLL contributed to study conception and design; data acquisition, data cleaning, analysis, and interpretation; drafting the article, revising it critically for important intellectual content and final approval of the version to be published. MLL takes responsibility for the integrity of the data and the accuracy of the data analysis and serves as principal author.
CMQ contributed to data acquisition, analysis and interpretation; drafting the article and revising it critically for important intellectual content; and final approval of the version to be published. CMQ takes responsibility for communicating with the Editorial Office and all other co-authors as corresponding author.
AOS and JDD contributed to study design; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published.
Corresponding author
Ethics declarations
Data sharing statement
Authors agree to make data and materials supporting the results presented in the paper available upon reasonable request.
Conflict of interest
MLL, CMQ, AOS and JDD ever received travel grants to attend meetings sponsored by pharmaceutical companies. JDD has been principal investigator in clinical trials sponsored by SPMSD, MSD, GSK and Pfizer. JDD acted as Advisor for GSK and SPMSD.
Additional information
Communicated by Tobias Tenenbaum
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
López-Lacort, M., Muñoz-Quiles, C., Díez-Domingo, J. et al. Effectiveness of self-financed rotavirus vaccines on acute gastroenteritis primary care episodes using real-world data in Spain: a propensity score–matched analysis of cohort study. Eur J Pediatr (2024). https://doi.org/10.1007/s00431-024-05536-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00431-024-05536-0