Abstract
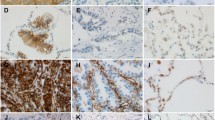
The aim of this study was to investigate the expression of integrins, their ligands, and integrin signaling-related molecules in a cohort of human primary non-small cell lung cancers (NSCLC). Formalin-fixed and paraffin-embedded tissue samples from 215 NSCLC were immunohistochemically stained using antibodies directed against αvβ3, αvβ5, αvβ6, αvβ8, αv, osteopontin, fibronectin, vitronectin, epidermal growth factor (EGFR), vascular endothelial growth factor receptor (VEGFR), and Ki67. Immunostaining of tumor, stroma, and endothelial cells was evaluated separately by quantity and intensity (tumor cells) or intensity (stroma and endothelial cells) expressed in an immunoreactivity score. We studied correlations between the staining patterns of the different markers and of marker expression with clinicopathological data and patient survival. In the majority of NSCLC, each marker was expressed in at least one tumor component. As expected, αv and αv integrin heterodimers were significantly co-expressed, as were integrins and EGFR. Vitronectin was expressed significantly more often in smaller (T-category) and in well-differentiated tumors; Ki67 index was higher in larger (T-category) and in poorly differentiated tumors. No significant correlation was found between any marker expression and gender, venous invasion, lymph vessel invasion, lymph node metastasis, or survival. Although integrin expression does not seem to be associated with indicators of progression of NSCLC, the expression of αvβ3 in 89 % and αvβ5 in 100 % of NSCLC is novel and merits to be further investigated.


Similar content being viewed by others
References
Herbst RS, Heymach JV, Lippman SM (2008) Molecular origins of cancer: lung cancer. N Engl J Med 13:1367–1380
Wu KH, House L, Liu WQ, Cho WCS (2012) Personalized targeted therapy for lung cancer. Int J Mol Sci 9:11471–11496
Max R, Gerritsen RR, Nooijen PT, Goodman SL, Sutter A, Keilholz U, Ruiter DJ, De Waal RM (1997) Immunohistochemical analysis of integrin alpha vbeta3 expression on tumor-associated vessels of human carcinomas. Int J Cancer 3:320–324
Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld R, Hu T, Klier G, Cheresh DA (1994) Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 7:1157–1164
Felding-Habermann B, Mueller BM, Romerdahl CA, Cheresh DA (1992) Involvement of integrin alpha V gene expression in human melanoma tumorigenicity. J Clin Invest 6:2018–2022
Barczyk M, Carracedo S, Gullberg D (2010) Integrins. Cell Tissue Res 1:269–280
Mitjans F, Sander D, Adan J, Sutter A, Martinez JM, Jaggle CS, Moyano JM, Kreysch HG, Piulats J, Goodman SL (1995) An anti-alpha v-integrin antibody that blocks integrin function inhibits the development of a human melanoma in nude mice. J Cell Sci 108:2825–2838
Takada Y, Ye X, Simon S (2007) The integrins. Genome Biol 5:215
Tabatabai G, Weller M, Nabors B, Picard M, Reardon D, Mikkelsen T, Ruegg C, Stupp R (2010) Targeting integrins in malignant glioma. Target Oncol 3:175–181
Desgrosellier JS, Cheresh DA (2010) Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer 1:9–22
Humphries JD, Byron A, Humphries MJ (2006) Integrin ligands at a glance. J Cell Sci 19:3901–3903
Marshall JF, Hart IR (1996) The role of alpha v-integrins in tumor progression and metastasis. Semin Cancer Biol 3:129–138
Plow EF, Haas TK, Zhang L, Loftus J, Smith JW (2000) Ligand binding to integrins. J Biol Chem 29:21785–21788
Goodman S, Grote HJ, Wilm C (2012) Matched rabbit monoclonal antibodies against av-series integrins reveal a novel avb3-LIBS epitope, and permit routine staining of archival paraffin samples of human tumors. Biol Open 1:329–340
Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ, Van Schil PE, Garg K, Austin JHM, Asamura H, Rusch VW, Hirsch FR, Scagliotti G, Mitsudomi T, Huber RM, Ishikawa Y, Jett J, Sanchez-Cespedes M, Sculier JP, Takahashi T, Tsuboi M, Vansteenkiste J, Wistuba I, Yang PC, Aberle D, Brambilla C, Flieder D, Franklin W, Gazdar A, Gould M, Hasleton P, Henderson D, Johnson B, Johnson D, Kerr K, Kuriyama K, Lee JS, Miller VA, Petersen I, Roggli V, Rosell R, Saijo N, Thunnissen E, Tsao M, Yankelewitz D (2011) International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2:244–285
Wittekind C, Oberschmid B (2010) TNM classification of malignant tumors 2010. Pathologe 5:333–338
Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC (2004) Pathology and genetics of tumors of the lung, pleura, thymus and heart. IARC, Lyon
Packeisen J, Korsching E, Herbst H, Boecker W, Buerger H (2003) Demystified… Tissue microarray technology. Mol Pathol 4:198–204
Simon E, Petke D, Böger C, Behrens HM, Warneke V, Ebert M, Röcken C (2012) The spatial distribution of LGR5(+) cells correlates with gastric cancer progression. PLoS One 7: e35486
Benjamini Y (2010) Discovering the false discovery rate. J R Stat Soc Series B Stat Methodol 72:405–416
Ye JQ, Findeis-Hosey JJ, Yang Q, McMahon LA, Yao JL, Li FQ, Xu HD (2011) Combination of napsin A and TTF-1 immunohistochemistry helps in differentiating primary lung adenocarcinoma from metastatic carcinoma in the lung. Appl Immunohistochem 4:313–317
Kumar CC, Malkowski M, Yin Z, Tanghetti E, Yaremko B, Nechuta T, Varner J, Liu M, Smith EM, Neustadt B, Presta M, Armstrong L (2001) Inhibition of angiogenesis and tumor growth by SCH221153, a dual alpha(v)beta3 and alpha(v)beta5 integrin receptor antagonist. Cancer Res 5:2232–2238
Mitjans F, Meyer T, Fittschen C, Goodman S, Jonczyk A, Marshall JF, Reyes G, Piulats J (2000) In vivo therapy of malignant melanoma by means of antagonists of alphav integrins. Int J Cancer 5:716–723
Oliveira-Ferrer L, Hauschild J, Fiedler W, Bokemeyer C, Nippgen J, Celik I, Schuch G (2008) Cilengitide induces cellular detachment and apoptosis in endothelial and glioma cells mediated by inhibition of FAK/src/AKT pathway. J Exp Clin Cancer Res 27:86
Albert JM, Cao C, Geng L, Leavitt L, Hallahan DE, Lu B (2006) Integrin alpha v beta 3 antagonist cilengitide enhances efficacy of radiotherapy in endothelial cell and non-small-cell lung cancer models. Int J Radiat Oncol Biol Phys 5:1536–1543
Burke PA, DeNardo SJ, Miers LA, Lamborn KR, Matzku S, DeNardo GL (2002) Cilengitide targeting of alpha(v)beta(3) integrin receptor synergizes with radioimmunotherapy to increase efficacy and apoptosis in breast cancer xenografts. Cancer Res 15:4263–4272
Reardon DA, Nabors LB, Stupp R, Mikkelsen T (2008) Cilengitide: an integrin-targeting arginine-glycine-aspartic acid peptide with promising activity for glioblastoma multiforme. Expert Opin Investig Drugs 8:1225–1235
Loges S, Butzal M, Otten J, Schweizer M, Fischer U, Bokemeyer C, Hossfeld DK, Schuch G, Fiedler W (2007) Cilengitide inhibits proliferation and differentiation of human endothelial progenitor cells in vitro. Biochem Biophys Res Commun 4:1016–1020
Carter A (2010) Integrins as target: first phase III trial launches, but questions remain. J Natl Cancer Inst 10:675–677
Reardon DA, Fink KL, Mikkelsen T, Cloughesy TF, O’Neill A, Plotkin S, Glantz M, Ravin P, Raizer JJ, Rich KM, Schiff D, Shapiro WR, Burdette-Radoux S, Dropcho EJ, Wittemer SM, Nippgen J, Picard M, Nabors LB (2008) Randomized phase II study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. J Clin Oncol 34:5610–5617
Manegold C, Vansteenkiste J, Cardenal F, Schutte W, Woll P, Ulsperger E, Ruter B, Picard M, Eckmayr J, von Pawel J (2009) Randomized phase II study of three doses of the integrin inhibitor cilengitide versus docetaxel as second-line treatment for patients (pts) with stage IV non-small cell lung cancer (NSCLC). Invest New Drugs 31:175–182
Smythe WR, Wasfi D, Bavaria JE, Albelda SM, Kaiser LR (1997) Loss of alpha v integrin expression and recurrence in node-negative lung carcinoma. Ann Thorac Surg 4:949–953
Sipos B, Henopp T, Kalthoff H (2010) Expression of functional forms of vb3 and vb5 integrins, laminin-5 and vitronectin in common human tumors: consequences for cilengitide targeting. Cancer Res Suppl 70:2031
Singh B, Fu CZ, Bhattacharya J (2000) Vascular expression of the alpha(v)beta(3)-integrin in lung and other organs. Am J Physiol Lung Cell Mol Physiol 1:L217–L226
Tuck AB, Chambers AF, Allan AL (2007) Osteopontin overexpression in breast cancer: knowledge gained and possible implications for clinical management. J Cell Biochem 4:859–868
Chambers AF, Wilson SM, Kerkvliet N, OMalley FP, Harris JF, Casson AG (1996) Osteopontin expression in lung cancer. Lung Cancer 3:311–323
Thalmann GN, Sikes RA, Devoll RE, Kiefer JA, Markwalder R, Klima I, Farach-Carson CM, Studer UE, Chung LWK (1999) Osteopontin: possible role in prostate cancer progression. Clin Cancer Res 8:2271–2277
Ue T, Yokozaki H, Kitadai Y, Yamamoto S, Yasui W, Ishikawa T, Tahara E (1998) Co-expression of osteopontin and CD44v9 in gastric cancer. Int J Cancer 2:127–132
Donati V, Boldrini L, Dell’Omodarme M, Prati MC, Faviana P, Camacci T, Lucchi M, Mussi A, Santoro M, Basolo F, Fontanini G (2005) Osteopontin expression and prognostic significance in non-small cell lung cancer. Clin Cancer Res 18:6459–6465
Zhang J, Takahashi K, Takahashi F, Shimizu K, Ohshita F, Kameda Y, Maeda K, Nishio K, Fukuchi Y (2001) Differential osteopontin expression in lung cancer. Cancer Lett 2:215–222
Hu Z, Lin DM, Yuan JS, Xiao T, Zhang HS, Sun WY, Han NJ, Ma Y, Di XB, Gao MX, Ma JF, Zhang JH, Cheng SJ, Gao YN (2005) Overexpression of osteopontin is associated with more aggressive phenotypes in human non-small cell lung cancer. Clin Cancer Res 13:4646–4652
Nakamura H, Kawasaki N, Taguchi M, Kabasawa K (2006) Survival impact of epidermal growth factor receptor overexpression in patients with non-small cell lung cancer: a meta-analysis. Thorax 2:140–145
Moro L, Dolce L, Cabodi S, Bergatto E, Erba EB, Smeriglio M, Turco E, Retta SF, Giuffrida MG, Venturino M, Godovac-Zimmermann J, Conti A, Schaefer E, Beguinot L, Tacchetti C, Gaggini P, Silengo L, Tarone G, Defilippi P (2002) Integrin-induced epidermal growth factor (EGF) receptor activation requires c-Src and p130Cas and leads to phosphorylation of specific EGF receptor tyrosines. J Biol Chem 11:9405–9414
Timke C, Fritz E, Roeder F, Abdollahi A, Debus J, Koch M, Goodman S, Huber PE (2010) Combining integrin inhibition (cilengitide), EGFR inhibition (cetuximab) and radiation in a pancreatic cancer model. Int J Radiat Oncol Biol Phys Supple 3:644
Martin B, Paesmans M, Mascaux C, Berghmans T, Lothaire P, Meert AP, Lafitte JJ, Sculier JP (2004) Ki-67 expression and patients survival in lung cancer: systematic review of the literature with meta-analysis. Br J Cancer 12:2018–2025
Jakobsen JN, Sorensen JB (2013) Clinical impact of ki-67 labeling index in non-small cell lung cancer. Lung Cancer 1:1–7
Warth A, Muley T, Meister M, Stenzinger A, Thomas M, Schirmacher P, Schnabel PA, Budczies J, Hoffmann H, Weichert W (2012) The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol 13:1438–1446
Yoshizawa A, Sumiyoshi S, Sonobe M, Kobayashi M, Fujimoto M, Kawakami F, Tsuruyama T, Travis WD, Date H, Haga H (2013) Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations analysis of 440 Japanese patients. J Thorac Oncol 1:52–61
Russell PA, Wainer Z, Wright GM, Daniels M, Conron M, Williams RA (2011) Does lung adenocarcinoma subtype predict patient survival? A clinicopathologic study based on the New International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol 9:1496–1504
Ilie MI, Hofman V, Bonnetaud C, Havet K, Lespinet-Fabre V, Coelle C, Gavric-Tanga V, Venissac N, Mouroux J, Hofman P (2010) Usefulness of tissue microarrays for assessment of protein expression, gene copy number and mutational status of EGFR in lung adenocarcinoma. Virchows Arch 4:483–495
Kawahara A, Yamamoto C, Nakashima K, Azuma K, Hattori S, Kashihara M, Aizawa H, Basaki Y, Kuwano M, Kage M, Mitsudomi T, Ono M (2010) Molecular diagnosis of activating EGFR mutations in non-small cell lung cancer using mutation-specific antibodies for immunohistochemical analysis. Clin Cancer Res 12:3163–3170
Kawahara A, Taira T, Azuma K, Tominaga M, Hattori S, Kawahara M, Abe H, Yamaguchi T, Akiba J, Takamori S, Hayashi A, Kage M (2012) A diagnostic algorithm using EGFR mutation-specific antibodies for rapid response EGFR-TKI treatment in patients with non-small cell lung cancer. Lung Cancer 1:39–44
Nakamura H, Mochizuki A, Shinmyo T, Ando K, Kurimoto N, Yokote K, Takagi M (2010) Immunohistochemical detection of mutated epidermal growth factor receptors in pulmonary adenocarcinoma. Anticancer Res 12:5233–5237
Buffet W, Geboes KP, Dehertogh G, Geboes K (2008) EGFR-immunohistochemistry in colorectal cancer and non-small cell lung cancer: comparison of 3 commercially available EGFR-antibodies. Acta Gastro-Enterologica Belg 2:213–218
Weber GF, Lett GS, Haubein NC (2010) Osteopontin is a marker for cancer aggressiveness and patient survival. Br J Cancer 6:861–869
Acknowledgments
We wish to thank Marten Rönckendorf for his excellent technical assistance and Christian Röder for the provision of follow-up data.
Conflict of interest
Simon L. Goodman is gainfully employed by Merck KGaA. All other authors declare that they have no conflict of interest. This work was supported by grants from Merck KGaA Germany.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
(PDF 35.1 kb)
Online Resource 2
(PDF 92.2 kb)
Rights and permissions
About this article
Cite this article
Böger, C., Kalthoff, H., Goodman, S.L. et al. Integrins and their ligands are expressed in non-small cell lung cancer but not correlated with parameters of disease progression. Virchows Arch 464, 69–78 (2014). https://doi.org/10.1007/s00428-013-1506-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-013-1506-1