Abstract
Background
The purpose of this research was to investigate a possible link between night shift work and the development of all-cause dementia and Alzheimer's disease (AD), as well as determine the contribution of night shift work, genetic susceptibility to AD.
Methods
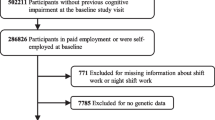
This study was conducted using the UK Biobank database. 245,570 participants with a mean follow-up length of 13.1 years were included. A Cox proportional hazards model was used to investigate the link between night shift work and the development of all-cause dementia or AD.
Results
We counted a total of 1248 participants with all-cause dementia. In the final multivariable adjusted model, the risk of dementia was highest in always night shift workers (HR 1.465, 95% CI 1.058–2.028, P = 0.022), followed by irregular shift workers (HR 1.197, 95% CI 1.026–1.396, P = 0.023). AD events were recorded in 474 participants during the follow-up period. After final multivariate adjustment of model, always night shift workers remained at the highest risk (HR 2.031, 95% CI 1.269–3.250, P = 0.003). Moreover, always night shift workers were associated with a higher risk of AD in both low, intermediate and high AD-GRS groups.
Conclusions
Always night shift work had a higher risk of developing all-cause dementia and AD. Irregular shift workers had a higher risk of developing all-cause dementia than no shift workers. Always night shift work had a higher AD risk, regardless of whether they had a high, intermediate or low AD-GRS.



Similar content being viewed by others
Data availability
Data supporting the results of this study are available from UK Biobank and are used under license.
References
Winblad B, Amouyel P, Andrieu S, Ballard C, Brayne C, Brodaty H, Cedazo-Minguez A, Dubois B, Edvardsson D, Feldman H, Fratiglioni L, Frisoni GB, Gauthier S, Georges J, Graff C, Iqbal K, Jessen F, Johansson G, Jonsson L, Kivipelto M, Knapp M, Mangialasche F, Melis R, Nordberg A, Rikkert MO, Qiu C, Sakmar TP, Scheltens P, Schneider LS, Sperling R, Tjernberg LO, Waldemar G, Wimo A, Zetterberg H (2016) Defeating Alzheimer’s disease and other dementias: a priority for European science and society. Lancet Neurol 15:455–532. https://doi.org/10.1016/S1474-4422(16)00062-4
Arvanitakis Z, Shah RC, Bennett DA (2019) Diagnosis and management of dementia: review. JAMA 322:1589–1599. https://doi.org/10.1001/jama.2019.4782
Ang ET, Tai YK, Lo SQ, Seet R, Soong TW (2010) Neurodegenerative diseases: exercising toward neurogenesis and neuroregeneration. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2010.00025
Kaplan GA, Turrell G, Lynch JW, Everson SA, Helkala EL, Salonen JT (2001) Childhood socioeconomic position and cognitive function in adulthood. Int J Epidemiol 30:256–263. https://doi.org/10.1093/ije/30.2.256
Farah MJ (2017) The neuroscience of socioeconomic status: correlates, causes, and consequences. Neuron 96:56–71. https://doi.org/10.1016/j.neuron.2017.08.034
Marden JR, Tchetgen TE, Kawachi I, Glymour MM (2017) Contribution of socioeconomic status at 3 life-course periods to late-life memory function and decline: early and late predictors of dementia risk. Am J Epidemiol 186:805–814. https://doi.org/10.1093/aje/kwx155
Strohmaier S, Devore EE, Zhang Y, Schernhammer ES (2018) A Review of data of findings on night shift work and the development of DM and CVD events: a synthesis of the proposed molecular mechanisms. Curr Diab Rep 18:132. https://doi.org/10.1007/s11892-018-1102-5
Gumenyuk V, Roth T, Drake CL (2012) Circadian phase, sleepiness, and light exposure assessment in night workers with and without shift work disorder. Chronobiol Int 29:928–936. https://doi.org/10.3109/07420528.2012.699356
Kim SM, Neuendorff N, Alaniz RC, Sun Y, Chapkin RS, Earnest DJ (2018) Shift work cycle-induced alterations of circadian rhythms potentiate the effects of high-fat diet on inflammation and metabolism. FASEB J 32:3085–3095. https://doi.org/10.1096/fj.201700784R
Vetter C, Dashti HS, Lane JM, Anderson SG, Schernhammer ES, Rutter MK, Saxena R, Scheer F (2018) Night shift work, genetic risk, and type 2 diabetes in the UK biobank. Diabetes Care 41:762–769. https://doi.org/10.2337/dc17-1933
Travis RC, Balkwill A, Fensom GK, Appleby PN, Reeves GK, Wang XS, Roddam AW, Gathani T, Peto R, Green J, Key TJ, Beral V (2016) Night shift work and breast cancer incidence: three prospective studies and meta-analysis of published studies. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djw169
Hublin C, Partinen M, Koskenvuo K, Silventoinen K, Koskenvuo M, Kaprio J (2010) Shift-work and cardiovascular disease: a population-based 22-year follow-up study. Eur J Epidemiol 25:315–323. https://doi.org/10.1007/s10654-010-9439-3
Skogstad M, Mamen A, Lunde LK, Ulvestad B, Matre D, Aass H, Ovstebo R, Nielsen P, Samuelsen KN, Skare O, Sirnes PA (2019) Shift work including night work and long working hours in industrial plants increases the risk of atherosclerosis. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph16030521
Wang N, Sun Y, Zhang H, Wang B, Chen C, Wang Y, Chen J, Tan X, Zhang J, Xia F, Qi L, Lu Y (2021) Long-term night shift work is associated with the risk of atrial fibrillation and coronary heart disease. Eur Heart J 42:4180–4188. https://doi.org/10.1093/eurheartj/ehab505
Ho FK, Celis-Morales C, Gray SR, Demou E, Mackay D, Welsh P, Katikireddi SV, Sattar N, Pell JP (2022) Association and pathways between shift work and cardiovascular disease: a prospective cohort study of 238 661 participants from UK Biobank. Int J Epidemiol 51:579–590. https://doi.org/10.1093/ije/dyab144
Nabe-Nielsen K, Garde AH, Ishtiak-Ahmed K, Gyntelberg F, Mortensen EL, Phung T, Rod NH, Waldemar G, Westendorp RG, Hansen AM (2017) Shift work, long working hours, and later risk of dementia: a long-term follow-up of the Copenhagen Male Study. Scand J Work Environ Health 43:569–577. https://doi.org/10.5271/sjweh.3660
Jorgensen JT, Hansen J, Westendorp R, Nabe-Nielsen K, Stayner LT, Simonsen MK, Andersen ZJ (2020) Shift work and incidence of dementia: a Danish nurse cohort study. Alzheimers Dement 16:1268–1279. https://doi.org/10.1002/alz.12126
Gan J, Wang XD, Shi Z, Yuan J, Zhang M, Liu S, Wang F, You Y, Jia P, Feng L, Xu J, Zhang J, Hu W, Chen Z, Ji Y (2021) The impact of rotating night shift work and daytime recharge on cognitive performance among retired nurses. Front Aging Neurosci 13:827772. https://doi.org/10.3389/fnagi.2021.827772
Mangialasche F, Kivipelto M, Solomon A, Fratiglioni L (2012) Dementia prevention: current epidemiological evidence and future perspective. Alzheimers Res Ther 4:6. https://doi.org/10.1186/alzrt104
Leso V, Caturano A, Vetrani I, Iavicoli I (2021) Shift or night shift work and dementia risk: a systematic review. Eur Rev Med Pharmacol Sci 25:222–232. https://doi.org/10.26355/eurrev_202101_24388
Bretsky P, Guralnik JM, Launer L, Albert M, Seeman TE (2003) The role of APOE-epsilon4 in longitudinal cognitive decline: MacArthur studies of successful aging. Neurology 60:1077–1081. https://doi.org/10.1212/01.wnl.0000055875.26908.24
Small BJ, Rosnick CB, Fratiglioni L, Backman L (2004) Apolipoprotein E and cognitive performance: a meta-analysis. Psychol Aging 19:592–600. https://doi.org/10.1037/0882-7974.19.4.592
Bendlin BB, Ries ML, Canu E, Sodhi A, Lazar M, Alexander AL, Carlsson CM, Sager MA, Asthana S, Johnson SC (2010) White matter is altered with parental family history of Alzheimer’s disease. Alzheimers Dement 6:394–403. https://doi.org/10.1016/j.jalz.2009.11.003
Wisdom NM, Callahan JL, Hawkins KA (2011) The effects of apolipoprotein E on non-impaired cognitive functioning: a meta-analysis. Neurobiol Aging 32:63–74. https://doi.org/10.1016/j.neurobiolaging.2009.02.003
Clark LR, Schiehser DM, Weissberger GH, Salmon DP, Delis DC, Bondi MW (2012) Specific measures of executive function predict cognitive decline in older adults. J Int Neuropsychol Soc 18:118–127. https://doi.org/10.1017/S1355617711001524
Wu WT, Li YJ, Feng AZ, Li L, Huang T, Xu AD, Lyu J (2021) Data mining in clinical big data: the frequently used databases, steps, and methodological models. Mil Med Res 8:44. https://doi.org/10.1186/s40779-021-00338-z
Yuan S, Huang X, Ma W, Yang R, Xu F, Han D, Huang T, Peng M, Xu A, Lyu J (2022) Associations of HDL-C/LDL-C with myocardial infarction, all-cause mortality, haemorrhagic stroke and ischaemic stroke: a longitudinal study based on 384 093 participants from the UK Biobank. Stroke Vasc NeurolDOI. https://doi.org/10.1136/svn-2022-001668
Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, Liu B, Matthews P, Ong G, Pell J, Silman A, Young A, Sprosen T, Peakman T, Collins R (2015) UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 12:e1001779. https://doi.org/10.1371/journal.pmed.1001779
Yang J, Li Y, Liu Q, Li L, Feng A, Wang T, Zheng S, Xu A, Lyu J (2020) Brief introduction of medical database and data mining technology in big data era. J Evid Based Med 13:57–69. https://doi.org/10.1111/jebm.12373
Maidstone R, Anderson SG, Ray DW, Rutter MK, Durrington HJ, Blaikley JF (2021) Shift work is associated with positive COVID-19 status in hospitalised patients. Thorax 76:601–606. https://doi.org/10.1136/thoraxjnl-2020-216651
Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, Motyer A, Vukcevic D, Delaneau O, O’Connell J, Cortes A, Welsh S, Young A, Effingham M, McVean G, Leslie S, Allen N, Donnelly P, Marchini J (2018) The UK Biobank resource with deep phenotyping and genomic data. Nature 562:203–209. https://doi.org/10.1038/s41586-018-0579-z
Marioni RE, Harris SE, Zhang Q, McRae AF, Hagenaars SP, Hill WD, Davies G, Ritchie CW, Gale CR, Starr JM, Goate AM, Porteous DJ, Yang J, Evans KL, Deary IJ, Wray NR, Visscher PM (2018) GWAS on family history of Alzheimer’s disease. Transl Psychiatry 8:99. https://doi.org/10.1038/s41398-018-0150-6
Jansen IE, Savage JE, Watanabe K, Bryois J, Williams DM, Steinberg S, Sealock J, Karlsson IK, Hagg S, Athanasiu L, Voyle N, Proitsi P, Witoelar A, Stringer S, Aarsland D, Almdahl IS, Andersen F, Bergh S, Bettella F, Bjornsson S, Braekhus A, Brathen G, de Leeuw C, Desikan RS, Djurovic S, Dumitrescu L, Fladby T, Hohman TJ, Jonsson PV, Kiddle SJ, Rongve A, Saltvedt I, Sando SB, Selbaek G, Shoai M, Skene NG, Snaedal J, Stordal E, Ulstein ID, Wang Y, White LR, Hardy J, Hjerling-Leffler J, Sullivan PF, van der Flier WM, Dobson R, Davis LK, Stefansson H, Stefansson K, Pedersen NL, Ripke S, Andreassen OA, Posthuma D (2019) Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat Genet 51:404–413. https://doi.org/10.1038/s41588-018-0311-9
Leng Y, Ackley SF, Glymour MM, Yaffe K, Brenowitz WD (2021) Genetic risk of Alzheimer’s disease and sleep duration in non-demented elders. Ann Neurol 89:177–181. https://doi.org/10.1002/ana.25910
Fan M, Sun D, Zhou T, Heianza Y, Lv J, Li L, Qi L (2020) Sleep patterns, genetic susceptibility, and incident cardiovascular disease: a prospective study of 385 292 UK biobank participants. Eur Heart J 41:1182–1189. https://doi.org/10.1093/eurheartj/ehz849
Haus E, Smolensky M (2006) Biological clocks and shift work: circadian dysregulation and potential long-term effects. Cancer Causes Control 17:489–500. https://doi.org/10.1007/s10552-005-9015-4
Bokenberger K, Sjolander A, Dahl AA, Karlsson IK, Akerstedt T, Pedersen NL (2018) Shift work and risk of incident dementia: a study of two population-based cohorts. Eur J Epidemiol 33:977–987. https://doi.org/10.1007/s10654-018-0430-8
Sharma A, Laurenti MC, Dalla MC, Varghese RT, Cobelli C, Rizza RA, Matveyenko A, Vella A (2017) Glucose metabolism during rotational shift-work in healthcare workers. Diabetologia 60:1483–1490. https://doi.org/10.1007/s00125-017-4317-0
Wei T, Li C, Heng Y, Gao X, Zhang G, Wang H, Zhao X, Meng Z, Zhang Y, Hou H (2020) Association between night-shift work and level of melatonin: systematic review and meta-analysis. Sleep Med 75:502–509. https://doi.org/10.1016/j.sleep.2020.09.018
Thomas J, Ooms SJ, Mentink LJ, Booij J, Olde RM, Overeem S, Kessels R, Claassen J (2020) Effects of long-term sleep disruption on cognitive function and brain amyloid-beta burden: a case-control study. Alzheimers Res Ther 12:101. https://doi.org/10.1186/s13195-020-00668-5
Nassan M, Videnovic A (2022) Circadian rhythms in neurodegenerative disorders. Nat Rev Neurol 18:7–24. https://doi.org/10.1038/s41582-021-00577-7
Akerstedt T (2003) Shift work and disturbed sleep/wakefulness. Occup Med (Lond) 53:89–94. https://doi.org/10.1093/occmed/kqg046
Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM (2009) Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 326:1005–1007. https://doi.org/10.1126/science.1180962
Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, Zhou Y, Wong DF, Ferrucci L, Resnick SM (2013) Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA Neurol 70:1537–1543. https://doi.org/10.1001/jamaneurol.2013.4258
Yang L, Luo Y, He L, Yin J, Li T, Liu S, Li D, Cheng X, Bai Y (2022) Shift work and the risk of cardiometabolic multimorbidity among patients with hypertension: a prospective cohort study of UK biobank. J Am Heart Assoc 11:e25936. https://doi.org/10.1161/JAHA.122.025936
Sun M, Feng W, Wang F, Li P, Li Z, Li M, Tse G, Vlaanderen J, Vermeulen R, Tse LA (2018) Meta-analysis on shift work and risks of specific obesity types. Obes Rev 19:28–40. https://doi.org/10.1111/obr.12621
Lane CA, Hardy J, Schott JM (2018) Alzheimer’s disease. Eur J Neurol 25:59–70. https://doi.org/10.1111/ene.13439
Yuan S, Wu W, Ma W, Huang X, Huang T, Peng M, Xu A, Lyu J (2022) Body mass index, genetic susceptibility, and Alzheimer’s disease: a longitudinal study based on 475,813 participants from the UK Biobank. J Transl Med 20:417. https://doi.org/10.1186/s12967-022-03621-2
Stephens ER, Sarangi A, Gude J (2022) Short sleep duration and dementia: a narrative review. Proc (Bayl Univ Med Cent) 35:328–331. https://doi.org/10.1080/08998280.2022.2026123
Bokenberger K, Strom P, Dahl AA, Johansson AL, Gatz M, Pedersen NL, Akerstedt T (2017) Association between sleep characteristics and incident dementia accounting for baseline cognitive status: a prospective population-based study. J Gerontol A Biol Sci Med Sci 72:134–139. https://doi.org/10.1093/gerona/glw127
Kyle SD, Sexton CE, Feige B, Luik AI, Lane J, Saxena R, Anderson SG, Bechtold DA, Dixon W, Little MA, Ray D, Riemann D, Espie CA, Rutter MK, Spiegelhalder K (2017) Sleep and cognitive performance: cross-sectional associations in the UK Biobank. Sleep Med 38:85–91. https://doi.org/10.1016/j.sleep.2017.07.001
Didikoglu A, Walker B, Maharani A, Pendleton N, Canal MM, Payton A, Gibson J, Brown T (2022) Associations between chronotype and employment status in a longitudinal study of an elderly population. Chronobiol Int 39:1118–1131. https://doi.org/10.1080/07420528.2022.2071158
Wu YH, Swaab DF (2005) The human pineal gland and melatonin in aging and Alzheimer’s disease. J Pineal Res 38:145–152. https://doi.org/10.1111/j.1600-079X.2004.00196.x
Hajali V, Andersen ML, Negah SS, Sheibani V (2019) Sex differences in sleep and sleep loss-induced cognitive deficits: the influence of gonadal hormones. Horm Behav 108:50–61. https://doi.org/10.1016/j.yhbeh.2018.12.013
Rangtell FH, Karamchedu S, Andersson P, Liethof L, Olaya BM, Lampola L, Schioth HB, Cedernaes J, Benedict C (2019) A single night of sleep loss impairs objective but not subjective working memory performance in a sex-dependent manner. J Sleep Res 28:e12651. https://doi.org/10.1111/jsr.12651
Marquie JC, Foret J (1999) Sleep, age, and shiftwork experience. J Sleep Res 8:297–304. https://doi.org/10.1046/j.1365-2869.1999.00170.x
Hajali V, Sheibani V, Esmaeili-Mahani S, Shabani M (2012) Female rats are more susceptible to the deleterious effects of paradoxical sleep deprivation on cognitive performance. Behav Brain Res 228:311–318. https://doi.org/10.1016/j.bbr.2011.12.008
Allen JS, Damasio H, Grabowski TJ, Bruss J, Zhang W (2003) Sexual dimorphism and asymmetries in the gray-white composition of the human cerebrum. Neuroimage 18:880–894. https://doi.org/10.1016/s1053-8119(03)00034-x
Madeira MD, Lieberman AR (1995) Sexual dimorphism in the mammalian limbic system. Prog Neurobiol 45:275–333. https://doi.org/10.1016/0301-0082(94)00052-j
Turner BB, Weaver DA (1985) Sexual dimorphism of glucocorticoid binding in rat brain. Brain Res 343:16–23. https://doi.org/10.1016/0006-8993(85)91153-9
Hinojosa-Laborde C, Chapa I, Lange D, Haywood JR (1999) Gender differences in sympathetic nervous system regulation. Clin Exp Pharmacol Physiol 26:122–126. https://doi.org/10.1046/j.1440-1681.1999.02995.x
Acknowledgements
We express our gratitude to the UK Biobank participants, the research and investigation team members, and the project development and management staff.
Funding
This study was funded by Guangdong Provincial Key Laboratory of Traditional Chinese Medicine Informatization (Grant No. 2021B1212040007).
Author information
Authors and Affiliations
Contributions
JL and AX conceptualised the research aims; YL, SY, XH, TH and ST participated in data analysis and interpretation; YL drafted the manuscript; SY and XH revised the manuscript; all authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest.
Ethical approval
The UK Biobank study was approved by the Northwest Multi-centre Research Ethics Committee. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent to participate
All participants agreed to participate and signed the informed consent.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ling, Y., Yuan, S., Huang, X. et al. The association of night shift work with the risk of all-cause dementia and Alzheimer's disease: a longitudinal study of 245,570 UK Biobank participants. J Neurol 270, 3499–3510 (2023). https://doi.org/10.1007/s00415-023-11672-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-11672-8