Abstract
Background
Although several progressive supranuclear palsy (PSP) phenotypes have recently been described, studies identifying cognitive and neuropsychiatric differences between them are lacking.
Methods
An extensive battery of cognitive and behavioural assessments was administered to 63 PSP patients, 25 PD patients with similar sociodemographic characteristics, and 25 healthy controls. We analysed differences in phenomenology, frequency and severity of cognitive and neuropsychiatric symptoms between PSP, PD and HC, and between PSP subtypes.
Results
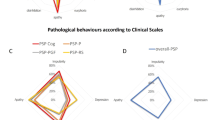
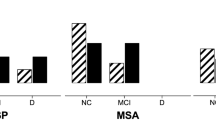
Regarding phenotypes, 64.6% met criteria for Richardson’s syndrome (PSP-RS), 10.7% PSP with predominant Parkinsonism (PSP-P), 10.7% with PSP progressive gait freezing (PSP-PGF), and 10.7% PSP with predominant speech/language disorder (PSP-SL). Impairment was more severe in the PSP group than in the PD and HC groups regarding motor scores, cognitive testing and neuropsychiatric scales. Cognitive testing did not clearly differentiate between PSP phenotypes, but PSP-RS and PSP-SL appeared to have more cognitive impairment than PSP-PGF and PSP-P, mainly due to an increased impairment in frontal executive domains. Regarding neuropsychiatric disturbances, no specific behavior was more common in any of the PSP subtypes.
Conclusion
Motor deficits delineate the phenotypes included in currently accepted MDS-PSP criteria. Cognition and behavioural disturbances are common in PSP and allow us to distinguish this disorder from other neurological diseases, but they do not differentiate between PSP phenotypes.
Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Nath U (2001) The prevalence of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome) in the UK. Brain 124(7):1438–1449. https://doi.org/10.1093/brain/124.7.1438
Coughlin D, Irwin DJ (2017) Emerging diagnostic and therapeutic strategies for tauopathies. Curr Neurol Neurosci Rep 17:9. https://doi.org/10.1007/s11910-017-0779-1
Boxer AL, Yu JT, Golbe LI, Litvan I, Lang AE, Höglinger GU (2017) Advances in progressive supranuclear palsy: new diagnostic criteria, biomarkers, and therapeutic approaches. Lancet Neurol 16(7):552–563. https://doi.org/10.1016/S1474-4422(17)30157-6
Cordato NJ, Halliday GM, Caine D, Morris JGL (2006) Comparison of motor, cognitive, and behavioral features in progressive supranuclear palsy and Parkinson’s disease. Mov Disord 21(5):632–638. https://doi.org/10.1002/mds.20779
Williams DR, Lees AJ (2009) Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges. Lancet Neurol 8(3):270–279. https://doi.org/10.1016/S1474-4422(09)70042-0
Osaki Y et al (2004) Accuracy of clinical diagnosis of progressive supranuclear palsy. Mov Disord 19(2):181–189. https://doi.org/10.1002/mds.10680
Respondek G et al (2014) The phenotypic spectrum of progressive supranuclear palsy: a retrospective multicenter study of 100 definite cases. Mov Disord 29(14):1758–1766. https://doi.org/10.1002/mds.26054
Respondek G, Hoglinger GU (2016) The phenotypic spectrum of progressive supranuclear palsy. Park Relat Disord 22:S34–S36. https://doi.org/10.1016/j.parkreldis.2015.09.041
Ali F et al (2019) Sensitivity and specificity of diagnostic criteria for progressive supranuclear palsy. Mov Disord 34(8):1144–1153. https://doi.org/10.1002/mds.27619
Kehagia AA, Barker RA, Robbins TW (2010) Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease. Lancet Neurol 9(12):1200–1213. https://doi.org/10.1016/S1474-4422(10)70212-X
Albert ML (1998) The subcortical dementia’ of progressive supranuclear palsy. Neurocase 4(2):95–98. https://doi.org/10.1093/neucas/4.2.95
Burrell JR, Hodges JR, Rowe JB (2014) Cognition in corticobasal syndrome and progressive supranuclear palsy: a review. Mov Disord 29(5):684–693. https://doi.org/10.1002/mds.25872
Parthimos TP, Schulpis KH (2020) The progressive supranuclear palsy: past and present aspects. Clin Gerontol 43(2):155–180. https://doi.org/10.1080/07317115.2019.1694115
Koga S et al (2017) Cognitive impairment in progressive supranuclear palsy is associated with tau burden. Mov Disord 32(12):1772–1779. https://doi.org/10.1002/mds.27198
Hughes AJ, Daniel SE, Blankson S, Lees AJ (1993) A clinicopathologic study of 100 cases of Parkinson’s Disease. Arch Neurol 50(2):140–148. https://doi.org/10.1001/archneur.1993.00540020018011
Rittman T, Coyle-Gilchrist IT, Rowe JB (2016) Managing cognition in progressive supranuclear palsy. Neurodegener Dis Manag 6(6):499–508. https://doi.org/10.2217/nmt-2016-0027
Kaat LD, Boon AJW, Kamphorst W, Ravid R, Duivenvoorden HJ, Van Swieten JC (2007) Frontal presentation in progressive supranuclear palsy. Neurology 69(8):723–729. https://doi.org/10.1212/01.wnl.0000267643.24870.26
Höglinger GU et al (2017) Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord 32(6):853–864. https://doi.org/10.1002/mds.26987
Gerstenecker A, Duff K, Mast B, Litvan I (2013) Behavioral abnormalities in progressive supranuclear palsy. Psychiatry Res 210(3):1205–1210. https://doi.org/10.1016/j.psychres.2013.08.045
Litvan I, Mega MS, Cummings JL, Fairbanks L (1996) Neuropsychiatric aspects of progressive supranuclear palsy. Neurology 47(5):1184–1189. https://doi.org/10.1212/WNL.47.5.1184
Lansdall CJ et al (2017) Apathy and impulsivity in frontotemporal lobar degeneration syndromes. Brain 140(6):1792–1807. https://doi.org/10.1093/brain/awx101
Picillo M et al (2019) Motor, cognitive and behavioral differences in MDS PSP phenotypes. J Neurol 266(7):1727–1735. https://doi.org/10.1007/s00415-019-09324-x
Grimm MJ et al (2019) How to apply the movement disorder society criteria for diagnosis of progressive supranuclear palsy. Mov Disord 34(8):1228–1232. https://doi.org/10.1002/mds.27666
Golbe LI, Ohman-Strickland PA (2007) A clinical rating scale for progressive supranuclear palsy. Brain 130(6):1552–1565. https://doi.org/10.1093/brain/awm032
Dubois B, Slachevsky A, Litvan I, Pillon B (2000) The FAB: a frontal assessment battery at bedside. Neurology 55(11):1621–1626. https://doi.org/10.1212/WNL.55.11.1621
Appollonio I et al (2005) The frontal assessment battery (FAB): Normative values in an Italian population sample. Neurol Sci 26(2):108–116. https://doi.org/10.1007/s10072-005-0443-4
Brown RG et al (2010) Cognitive impairment in patients with multiple system atrophy and progressive supranuclear palsy. Brain 133(8):2382–2393. https://doi.org/10.1093/brain/awq158
Caso F et al (2016) Cognitive impairment in progressive supranuclear palsy-Richardson’s syndrome is related to white matter damage. Park Relat Disord 31:65–71. https://doi.org/10.1016/j.parkreldis.2016.07.007
Kobylecki C et al (2015) Cognitive–behavioural features of progressive supranuclear palsy syndrome overlap with frontotemporal dementia. J Neurol 262(4):916–922. https://doi.org/10.1007/s00415-015-7657-z
Duff K, McDermott D, Luong D, Randolph C, Boxer AL (2019) Cognitive deficits in progressive supranuclear palsy on the repeatable battery for the assessment of neuropsychological status. J Clin Exp Neuropsychol 41(5):469–475. https://doi.org/10.1080/13803395.2019.1572073
Duff K, Randolph C, Boxer AL (2020) Cognitive decline on the repeatable battery for the assessment of neuropsychological status in progressive supranuclear palsy. Clin Neuropsychol 34(3):529–540. https://doi.org/10.1080/13854046.2019.1670865
Fiorenzato E, Antonini A, Camparini V, Weis L, Semenza C, Biundo R (2019) Characteristics and progression of cognitive deficits in progressive supranuclear palsy vs. multiple system atrophy and Parkinson’s disease. J Neural Trans 126(11):1437–1445. https://doi.org/10.1007/s00702-019-02065-1
Shoeibi A et al (2019) Are the International Parkinson disease and movement disorder society progressive supranuclear palsy (IPMDS-PSP) diagnostic criteria accurate enough to differentiate common PSP phenotypes? Park Relat Disord 69:34–39. https://doi.org/10.1016/j.parkreldis.2019.10.012
Santangelo G, Cuoco S, Pellecchia MT, Erro R, Barone P, Picillo M (2018) Comparative cognitive and neuropsychiatric profiles between Parkinson’s disease, multiple system atrophy and progressive supranuclear palsy. J Neurol 265(11):2602–2613. https://doi.org/10.1007/s00415-018-9038-x
Pilotto A et al (2017) Mild cognitive impairment and progression to dementia in progressive supranuclear palsy. Neurodegener Dis 17(6):286–291. https://doi.org/10.1159/000479110
Borroni B, Alberici A, Agosti C, Cosseddu M, Padovani A (2009) Pattern of behavioral disturbances in corticobasal degeneration syndrome and progressive supranuclear palsy. Int Psychogeriatrics 21(3):463–468. https://doi.org/10.1017/S1041610209008862
Erro R, Barone P, Moccia M, Amboni M, Vitale C (2013) Abnormal eating behaviors in progressive supranuclear palsy. Eur J Neurol 20(3):47–48. https://doi.org/10.1111/ene.12077
O’Keeffe FM et al (2007) Loss of insight in frontotemporal dementia, corticobasal degeneration and progressive supranuclear palsy. Brain 130(3):753–764. https://doi.org/10.1093/brain/awl367
Plutino A et al (2020) Insight in frontotemporal dementia and progressive supranuclear palsy. Neurol Sci. https://doi.org/10.1007/s10072-020-04290-z
Jabbari E et al (2020) Diagnosis across the spectrum of progressive supranuclear palsy and corticobasal syndrome. JAMA Neurol 77(3):377–387. https://doi.org/10.1001/jamaneurol.2019.4347
Martí-Andrés G et al (2020) Multicenter validation of metabolic abnormalities related to PSP according to the MDS-PSP criteria. Mov Disord 4:1–11. https://doi.org/10.1002/mds.28217
Acknowledgments
The authors thank all the patients and their families for their participation in this study.
Funding
In the last 12 months, Andrea Horta-Barba received a salary from the Research Institute of Sant Pau and received compensation for speaker activities from UCB and AbbVie. Javier Pagonabarraga has received honoraria as speaker or Member of Advisory Board for Zambon, Bial, UCB, Allergan, Abbvie, and Ipsen. Saül Martínez-Horta received a salary from the Research Institute of Sant Pau, has been awarded a grant from the Huntington’s Disease Society of America and received compensation for speaker activities from UCB, AbbVie and Roche. Laura Busteed is studying her Master of Science and has not received honoraria. Berta Pascual-Sedano received compensation for speaker activities from Abbvie, UCB and Ferrer. Ignacio Illán-Gala is supported by the Global Brain Health Institute (Atlantic Fellow for Equity in Brain Health). Juan Marín-Lahoz received a salary from the PERIS of the Generalitat of Catalonia. Ignacio Aracil-Bolaños has received a salary from the Research Institute of Sant Pau and research support from Carlos III Institute Research Grant. Jesús Pérez-Pérez is supported by a research grant from the Government of Spain (ISCIII). Frederic Sampedro is supported by a research grant from the Government of Spain (ISCIII). Helena Bejr-Kasem has received a salary from a pre-doctoral grant awarded by the Spanish Government (ISCIII), from the Research Institute of Sant Pau and received compensation for speaker activities in scientific meetings supported by Zambon, and non-financial support for congress attendance from Abbvie, Zambon and Allergan. Jaime Kulisevsky has received public research support from CIBERNED and Carlos III Institute, unrestrictive research support from Zambon and TEVA, and honoraria for lecturing and/or consulting from Zambon and TEVA.
Author information
Authors and Affiliations
Contributions
AH-B: research project conception, organization, execution, design. Formal analysis. Writing – original draft. JP: research project conception, organization, execution, design. Formal analysis. Writing – original draft. SM-H: research project conception, design. Formal analysis. Writing – review and critique. LB: research project organization and execution. Writing – review and critique. BP-S: research project organization and execution. Writing – review and critique. II-G: research project organization and execution. Writing – review and critique. JM-L: research project organization and execution. Writing – review and critique. IA-B: research project organization and execution. Writing – review and critique. JP-P: research project organization and execution. Writing – review and critique. FS: statistical analysis execution. Writing – review and critique. HB-K: research project organization and execution. Writing – review and critique. JK: research project conception, organization, design. Formal analysis. Writing – original draft.
Corresponding authors
Ethics declarations
Conflicts of interest
Nothing to report.
Ethical standard
The study has been approved by the ethical committee of Sant Pau Hospital (Barcelona, Spain). Written informed consent was obtained from all participants and the study was conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Horta-Barba, A., Pagonabarraga, J., Martínez-Horta, S. et al. Cognitive and behavioral profile of progressive supranuclear palsy and its phenotypes. J Neurol 268, 3400–3408 (2021). https://doi.org/10.1007/s00415-021-10511-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-021-10511-y