Abstract
Objectives
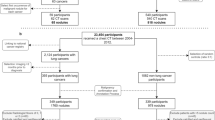
To establish deep learning models for malignancy risk estimation of sub-centimeter pulmonary nodules incidentally detected by chest CT and managed in clinical settings.
Materials and methods
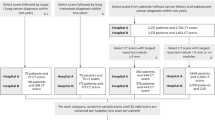
Four deep learning models were trained using CT images of sub-centimeter pulmonary nodules from West China Hospital, internally tested, and externally validated on three cohorts. The four models respectively learned 3D deep features from the baseline whole lung region, baseline image patch where the nodule located, baseline nodule box, and baseline plus follow-up nodule boxes. All regions of interest were automatically segmented except that the nodule boxes were additionally manually checked. The performance of models was compared with each other and that of three respiratory clinicians.
Results
There were 1822 nodules (981 malignant) in the training set, 806 (416 malignant) in the testing set, and 357 (253 malignant) totally in the external sets. The area under the curve (AUC) in the testing set was 0.754, 0.855, 0.928, and 0.942, respectively, for models derived from baseline whole lung, image patch, nodule box, and the baseline plus follow-up nodule boxes. When baseline models externally validated (follow-up images not available), the nodule-box model outperformed the other two with AUC being 0.808, 0.848, and 0.939 respectively in the three external datasets. The resident, junior, and senior clinicians achieved an accuracy of 67.0%, 82.5%, and 90.0%, respectively, in the testing set. The follow-up model performed comparably to the senior clinician.
Conclusion
The deep learning algorithms solely mining nodule information can efficiently predict malignancy of incidental sub-centimeter pulmonary nodules.
Clinical relevance statement
The established models may be valuable for supporting clinicians in routine clinical practice, potentially reducing the number of unnecessary examinations and also delays in diagnosis.
Key Points
• According to different regions of interest, four deep learning models were developed and compared to evaluate the malignancy of sub-centimeter pulmonary nodules by CT images.
• The models derived from baseline nodule box or baseline plus follow-up nodule boxes demonstrated sufficient diagnostic accuracy (86.4% and 90.4% in the testing set), outperforming the respiratory resident (67.0%) and junior clinician (82.5%).
• The proposed deep learning methods may aid clinicians in optimizing follow-up recommendations for sub-centimeter pulmonary nodules and may lead to fewer unnecessary diagnostic interventions.






Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the curve
- CT:
-
Computed tomography
- DCA:
-
Decision curve analysis
- KMU:
-
First Affiliated Hospital of Kunming Medical University
- NSMC:
-
Affiliated Hospital of North Sichuan Medical College
- ROC curve:
-
Receiver operating characteristic curve
- SCH:
-
Suining Central Hospital
- WCH:
-
West China Hospital of Sichuan University
References
Walter K (2021) Pulmonary nodules. JAMA 326(15):1544
Au-Yong ITH, Hamilton W, Rawlinson J, Baldwin DR (2020) Pulmonary nodules. BMJ 371:m3673
National Lung Screening Trial Research Team (2013) Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 368(21):1980–1991
Oke JL, Pickup LC, Declerck J et al (2018) Development and validation of clinical prediction models to risk stratify patients presenting with small pulmonary nodules: a research protocol. Diagn Progn Res 2:22
MacMahon H, Austin JHM, Gamsu G et al (2005) Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 237(2):395–400
Libby DM, Smith JP, Altorki NK, Pasmantier MW, Yankelevitz D, Henschke CI (2004) Managing the small pulmonary nodule discovered by CT. Chest 125(4):1522–1529
Madsen PH, Holdgaard PC, Christensen JB, Høilund-Carlsen PF (2016) Clinical utility of F-18 FDG PET-CT in the initial evaluation of lung cancer. Eur J Nucl Med Mol Imaging 43(11):2084–2097
Lung CT Screening Reporting & Data System (Lung-RADS®), American College of Radiology (2023) Available via https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Lung-Rads. Accessed 1 Jun 2023
MacMahon H, Naidich DP, Goo JM et al (2017) Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology 284(1):228–243
van Riel SJ, Sánchez CI, Bankier AA et al (2015) Observer variability for classification of pulmonary nodules on low-dose CT images and its effect on nodule management. Radiology 277(3):863–871
McWilliams A, Tammemagi MC, Mayo JR et al (2013) Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med 369(10):910–919
Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES (1997) The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med 157(8):849–855
Bi WL, Hosny A, Schabath MB et al (2019) Artificial intelligence in cancer imaging: clinical challenges and applications. CA Cancer J Clin 69(2):127–157
Xu Y, Lu L, Sun SH et al (2021) Effect of CT image acquisition parameters on diagnostic performance of radiomics in predicting malignancy of pulmonary nodules of different sizes. Eur Radiol 32(3):1517–1527
Venkadesh KV, Setio AAA, Schreuder A et al (2021) Deep learning for malignancy risk estimation of pulmonary nodules detected at low-dose screening CT. Radiology 300(2):438–447
Mazzone PJ, Lam L (2022) Evaluating the patient with a pulmonary nodule. JAMA 327(3):264–273
Wu J, Xia Y, Wang X et al (2023) uRP: an integrated research platform for one-stop analysis of medical images. Front Radiol 3:1153784
He K, Zhang X, Ren S, Sun J (2016) Deep residual learning for image recognition. Proceedings of the IEEE conference on computer vision and pattern recognition, pp 770–778
Lin TY, Goyal P, Girshick R, He KM, Dollar P (2020) Focal loss for dense object detection. IEEE Trans Pattern Anal Mach Intell 42(2):318–327
Gao F, Sun YL, Zhang GZ, Zheng XP, Li M, Hua YQ (2019) CT characterization of different pathological types of subcentimeter pulmonary ground-glass nodular lesions. Br J Radiol 92(1094):20180204
Ohtsuka T (2003) Radiological examination for peripheral lung cancers and benign nodules less than 10 mm. Lung Cancer 42(3):291–296
Zhu Y, Yip R, You N, Henschke CI, Yankelevitz DF (2020) Management of nodules attached to the costal pleura at low-dose CT screening for lung cancer. Radiology 297(3):710–718
Takashima S, Sone S, Li F et al (2003) Small solitary pulmonary nodules (<= 1 cm) detected at population-based CT screening for lung cancer: reliable high-resolution CT features of benign lesions. AJR Am J Roentgenol 180(4):955–964
Chen C, Geng Q, Song G et al (2023) A comprehensive nomogram combining CT-based radiomics with clinical features for differentiation of benign and malignant lung subcentimeter solid nodules. Front Oncol 13:1066360
Chen X, Feng B, Chen Y et al (2020) A CT-based radiomics nomogram for prediction of lung adenocarcinomas and granulomatous lesions in patient with solitary sub-centimeter solid nodules. Cancer Imaging 20(1):45
Hu X, Ye W, Li Z et al (2020) Non-invasive evaluation for benign and malignant subcentimeter pulmonary ground-glass nodules (≤1 cm) based on CT texture analysis. Br J Radiol 93(1114):20190762
Zhao W, Yang J, Sun Y, Li C, Wu W, Jin L (2018) 3D Deep learning from CT scans predicts tumor invasiveness of subcentimeter pulmonary adenocarcinomas. Cancer Res 78(24):6881–6889
Shin KE, Lee KS, Yi CA, Chung MJ, Shin M-H, Choi Y-H (2014) Subcentimeter lung nodules stable for 2 years at LDCT: long-term follow-up using volumetry. Respirology 19(6):921–928
Slattery MM, Foley C, Kenny D, Costello RW, Logan PM, Lee MJ (2012) Long-term follow-up of non-calcified pulmonary nodules (<10 mm) identified during low-dose CT screening for lung cancer. Eur Radiol 22(9):1923–1928
Kakinuma R, Muramatsu Y, Kusumoto M et al (2015) Solitary pure ground-glass nodules 5 mm or smaller: frequency of growth. Radiology 276(3):873–882
Cho J, Kim ES, Kim SJ et al (2016) Long-term follow-up of small pulmonary ground-glass nodules stable for 3 years: implications of the proper follow-up period and risk factors for subsequent growth. J Thorac Oncol 11(9):1453–1459
Lee HW, Jin K-N, Lee J-K et al (2019) Long-term follow-up of ground-glass nodules after 5 years of stability. J Thorac Oncol 14(8):1370–1377
Qi LL, Wu BT, Tang W et al (2020) Long-term follow-up of persistent pulmonary pure ground-glass nodules with deep learning-assisted nodule segmentation. Eur Radiol 30(2):744–755
Takahashi S, Tanaka N, Okimoto T et al (2011) Long term follow-up for small pure ground-glass nodules: implications of determining an optimum follow-up period and high-resolution CT findings to predict the growth of nodules. Jpn J Radiol 30(3):206–217
Xue LM, Li Y, Zhang Y et al (2021) A predictive nomogram for two-year growth of CT-indeterminate small pulmonary nodules. Eur Radiol 32(4):2672–2682
Wang S, Yu H, Gan Y et al (2022) Mining whole-lung information by artificial intelligence for predicting EGFR genotype and targeted therapy response in lung cancer: a multicohort study. Lancet Digit Health 4(5):e309–e319
Funding
This research is supported by Key R & D plan of Sichuan Provincial Department of science and technology (2021YFS0072), Natural Science Foundation of Sichuan Province (2022NSFSC0785), Key R & D program of Sichuan-Chongqing of the Chongqing Science and Technology Commission (CSTB2022TIAD-CUX0001), and Full-time Postdoctoral Program of Sichuan University (2023SCU12048).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Weimin Li.
Conflict of interest
The authors declare no conflict of interest. Qing Zhou is an employee of Shanghai United Imaging Intelligence Co., Ltd.
Statistics and biometry
The authors Zhang Rui and Ying Wei have significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board because this was a retrospective study and patients’ anonymity was carefully protected.
Ethical approval
All methods were performed in accordance with the Declaration of Helsinki. The study was approved by the institutional review boards of the West China Hospital of Sichuan University, Affiliated Hospital of North Sichuan Medical College, First Affiliated Hospital of Kunming Medical University, and Suining Central Hospital.
Study subjects or cohorts overlap
No study subjects or cohorts have been previously reported.
Methodology
• retrospective
• diagnostic study
• multicenter study
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rui Zhang, Ying Wei, and Denian Wang contributed equally to this work.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, R., Wei, Y., Wang, D. et al. Deep learning for malignancy risk estimation of incidental sub-centimeter pulmonary nodules on CT images. Eur Radiol (2023). https://doi.org/10.1007/s00330-023-10518-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00330-023-10518-1