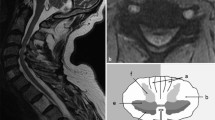
Abstract
Objectives
To investigate the association between spinal cord perfusion and microstructural damage in CSM patients who underwent cervical laminoplasty using MR dynamic susceptibility contrast (DSC), diffusion tensor imaging (DTI), and neurite orientation dispersion and density imaging (NODDI) techniques.
Methods
A follow-up cohort study was conducted with 53 consecutively recruited CSM patients who had undergone cervical laminoplasty 12–14 months after the surgery from April 2016 to December 2016. Twenty-one aged-matched healthy volunteers were recruited as controls. For each patient, decompressed spinal cord levels were imaged on a 3.0-T MRI scanner by diffusion and DSC sequences to quantify the degrees of microstructural damage and perfusion conditions, respectively. The diffusion data were analyzed by DTI and NODDI models to produce diffusion metrics. Classic indicator dilution model was used to quantify the DSC metrics. Mann–Whitney U test was performed for comparison of diffusion metrics between patients and healthy controls. Pearson correlation was used to explore the associations between the metrics of spinal cord perfusion and microstructural damage.
Results
DTI metrics, neurite density, and isotropic volume fraction had significant differences between postoperative patients and healthy controls. Pearson correlation test showed that SCBV was significantly positively correlated with RD, MD, and ODI, and negatively correlated with FA and NDI. SCBF was found to be significantly positively correlated with RD and MD, and negatively correlated with FA.
Conclusions
Increased spinal cord perfusion quantified by DSC is associated with microstructural damage assessed by diffusion MRI in CSM patients who underwent cervical laminoplasty.
Clinical relevance statement
This study found that the spinal cord perfusion is associated with microstructural damage in postoperative cervical spondylotic myelopathy patients, indicating that high perfusion may play a role in the pathophysiological process of cervical spondylotic myelopathy and deserves more attention.
Key Points
• Spinal cord microstructural damage can be persistent despite the compression had been relieved 12–14 months after the cervical laminoplasty in cervical spondylotic myelopathy (CSM) patients.
• Spinal cord perfusion is associated with microstructural damage in CSM patients after the cervical laminoplasty.
• Inflammation in the decompressed spinal cord may be a cause of increased perfusion and is associated with microstructural damage during the recovery period of CSM.



Similar content being viewed by others
Abbreviations
- AD:
-
Axial diffusivity
- CSM:
-
Cervical spondylotic myelopathy
- DSC:
-
Dynamic susceptibility contrast
- DTI:
-
Diffusion tensor imaging
- FA:
-
Fractional anisotropy
- MD:
-
Mean diffusivity
- MTT:
-
Mean transit time
- NDI:
-
Neurite density index
- NODDI:
-
Neurite orientation dispersion and density imaging
- ODI:
-
Orientation dispersion index
- RD:
-
Radial diffusivity
- SCBF:
-
Spinal cord blood flow
- SCBV:
-
Spinal cord blood volume
- Viso:
-
Isotropic volume fraction
References
Ellingson BM, Salamon N, Holly LT (2015) Advances in MR imaging for cervical spondylotic myelopathy. Eur Spine J 24(2):197–208. https://doi.org/10.1007/s00586-013-2915-1
Karadimas SK, Gatzounis G, Fehlings MG (2015) Pathobiology of cervical spondylotic myelopathy. Eur Spine J 24(2):132–138. https://doi.org/10.1007/s00586-014-3264-4
Kalsi-Ryan S, Karadimas SK, Fehlings MG (2013) Cervical spondylotic myelopathy the clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist 19(4):409–421. https://doi.org/10.1177/1073858412467377
Tetreault L, Goldstein CL, Arnold P et al (2015) Degenerative cervical myelopathy: a spectrum of related disorders affecting the aging spine. Neurosurgery 77(suppl_1):S51–S67. https://doi.org/10.1227/NEU.0000000000000951
Viallon M, Cuvinciuc V, Delattre B et al (2015) State-of-the-art MRI techniques in neuroradiology: principles, pitfalls, and clinical applications. Neuroradiology 57(10):441–467. https://doi.org/10.1007/s00234-015-1500-1
Aditya V, Micheal J, Gerald E et al (2013) Diffusion tensor imaging of the spinal cord: a review. Coluna/Columna 12:64–69. https://doi.org/10.1590/S1808-18512013000100014
Kara B, Celik A, Karadereler S et al (2011) The role of DTI in early detection of cervical spondylotic myelopathy: a preliminary study with 3-T MRI. Neuroradiology 53(8):609–616. https://doi.org/10.1007/s00234-011-0844-4
Budzik JF, Balbi V, Thuc VL, Duhamel A, Assaker R, Cotten A (2011) Diffusion tensor imaging and fibre tracking in cervical spondylotic myelopathy. Eur Radiol 21(2):426–433. https://doi.org/10.1007/s00330-010-1927-z
Gao SJ, Yuan X, Jiang XY et al (2013) Correlation study of 3T-MR-DTI measurements and clinical symptoms of cervical spondylotic myelopathy. Eur J Radiol 82(11):1940–1945. https://doi.org/10.1016/j.ejrad.2013.06.011
Ma X, Han X, Jiang W et al (2018) A follow-up study of postoperative DCM patients using diffusion MRI with DTI and NODDI. Spine (Phila Pa 1976) 43(15):E898–E904. https://doi.org/10.1097/BRS.0000000000002541
Jiang W, Han X, Guo H et al (2018) Usefulness of conventional magnetic resonance imaging, diffusion tensor imaging and neurite orientation dispersion and density imaging in evaluating postoperative function in patients with cervical spondylotic myelopathy. J Orthop Transl 15:59–69. https://doi.org/10.1016/j.jot.2018.08.006
Ellingson BM, Salamon N, Grinstead JW, Holly LT (2014) Diffusion tensor imaging predicts functional impairment in mild-to-moderate cervical spondylotic myelopathy. Spine J 14(11):2589–2597. https://doi.org/10.1016/j.spinee.2014.02.027
Jones JG, Cen SY, Lebel RM, Hsieh PC, Law M (2013) Diffusion tensor imaging correlates with the clinical assessment of disease severity in cervical spondylotic myelopathy and predicts outcome following surgery. AJNR Am J Neuroradiol 34(2):471–478. https://doi.org/10.3174/ajnr.A3199
Han X, Ma X, Li D et al (2020) The evaluation and prediction of laminoplasty surgery outcome in patients with degenerative cervical myelopathy using diffusion tensor MRI. AJNR Am J Neuroradiol 41(9):1745–1753. https://doi.org/10.3174/ajnr.A6705
Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC (2012) NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 61(4):1000–1016. https://doi.org/10.1016/j.neuroimage.2012.03.072
Okita G, Ohba T, Takamura T et al (2018) Application of neurite orientation dispersion and density imaging or diffusion tensor imaging to quantify the severity of cervical spondylotic myelopathy and to assess postoperative neurologic recovery. Spine J 18(2):268–275. https://doi.org/10.1016/j.spinee.2017.07.007
Cohen-Adad J (2018) Microstructural imaging in the spinal cord and validation strategies. Neuroimage 182:169–183. https://doi.org/10.1016/j.neuroimage.2018.04.009
Smith AM, Grandin CB, Duprez T, Mataigne F, Cosnard G (2000) Whole brain quantitative CBF, CBV, and MTT measurements using MRI bolus tracking: implementation and application to data acquired from hyperacute stroke patients. J Magn Reson Imaging 12(3):400–410. https://doi.org/10.1002/1522-2586(200009)12:3%3c400::AID-JMRI5%3e3.0.CO;2-C
Rempp KA, Brix G, Wenz F, Becker CR, Gückel F, Lorenz WJ (1994) Quantification of regional cerebral blood flow and volume with dynamic susceptibility contrast-enhanced MR imaging. Radiology 193:637–641. https://doi.org/10.1148/radiology.193.3.7972800
Essock-Burns E, Phillips JJ, Molinaro AM et al (2013) Comparison of DSC-MRI post-processing techniques in predicting microvascular histopathology in patients newly diagnosed with GBM. J Magn Reson Imaging 38(2):388–400. https://doi.org/10.1002/jmri.23982
Paulson ES, Schmainda KM (2008) Comparison of dynamic susceptibility-weighted contrast-enhanced MR methods: recommendations for measuring relative cerebral blood volume in brain tumors. Radiology 249(2):601–613. https://doi.org/10.1148/radiol.2492071659
Bauer AH, Erly W, Moser FG, Maya M, Nael K (2015) Differentiation of solitary brain metastasis from glioblastoma multiforme: a predictive multiparametric approach using combined MR diffusion and perfusion. Neuroradiology 57(7):697–703. https://doi.org/10.1007/s00234-015-1524-6
Wang S, Martinez-Lage M, Sakai Y et al (2015) Differentiating tumor progression from pseudoprogression in patients with glioblastomas using diffusion tensor imaging and dynamic susceptibility contrast MRI. AJNR Am J Neuroradiol 37(1):28–36. https://doi.org/10.3174/ajnr.a4474
Tournier JD, Smith R, Raffelt D et al (2019) MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage 202:116137. https://doi.org/10.1016/j.neuroimage.2019.116137
Veraart J, Novikov DS, Christiaens D, Ades-Aron B, Sijbers J, Fieremans E (2016) Denoising of diffusion MRI using random matrix theory. Neuroimage 142:394–406. https://doi.org/10.1016/j.neuroimage.2016.08.016
Veraart J, Fieremans E, Novikov DS (2016) Diffusion MRI noise mapping using random matrix theory. Magn Reson Med 76(5):1582–1593. https://doi.org/10.1002/mrm.26059
Kellner E, Dhital B, Kiselev VG, Reisert M (2016) Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn Reson Med 76(5):1574–1581. https://doi.org/10.1002/mrm.26054
Andersson JLR, Sotiropoulos SN (2016) An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 125:1063–1078. https://doi.org/10.1016/j.neuroimage.2015.10.019
Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012) FSL. Neuroimage 62(2):782–790. https://doi.org/10.1016/j.neuroimage.2011.09.015
De Leener B, Levy S, Dupont SM et al (2017) SCT: spinal cord toolbox, an open-source software for processing spinal cord MRI data. Neuroimage 145:24–43. https://doi.org/10.1016/j.neuroimage.2016.10.009
Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S (2006) DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Meth Prog Bio 81(2):106–116. https://doi.org/10.1016/j.cmpb.2005.08.004
Carroll TJ, Rowley HA, Haughton VM (2003) Automatic calculation of the arterial input function for cerebral perfusion imaging with MR imaging. Radiology 227(2):593–600. https://doi.org/10.1148/radiol.2272020092
Ranzenberger LR, Snyder T (2022) Diffusion tensor imaging. In: StatPearls. Treasure Island (FL): StatPearls Publishing; July 26
Lu H, Law M, Ge Y et al (2010) Quantitative measurement of spinal cord blood volume in humans using vascular-space-occupancy MRI. NMR Biomed 21(3):226–232. https://doi.org/10.1002/nbm.1185
Beattie MS, Manley GT (2011) Tight squeeze, slow burn: inflammation and the aetiology of cervical myelopathy. Brain 134(5):1259–1261. https://doi.org/10.1093/brain/awr088
Yu WR, Liu T, Kiehl TR, Fehlings MG (2011) Human neuropathological and animal model evidence supporting a role for Fas-mediated apoptosis and inflammation in cervical spondylotic myelopathy. Brain 134(5):1277–1292. https://doi.org/10.1093/brain/awr090
Yu WR, Baptiste DC, Liu T, Odrobina E, Stanisz GJ, Fehlings MG (2009) Molecular mechanisms of spinal cord dysfunction and cell death in the spinal hyperostotic mouse: implications for the pathophysiology of human cervical spondylotic myelopathy. Neurobiol Dis 33(2):149–163. https://doi.org/10.1016/j.nbd.2008.09.024
Kerwin W, Hooker A, Spilker M et al (2003) Quantitative magnetic resonance imaging analysis of neovasculature volume in carotid atherosclerotic plaque. Circulation 107(6):851–856. https://doi.org/10.1161/01.CIR.0000048145.52309.31
Tetreault LA, Zhu MP, Howard RM et al (2019) The discrepancy between functional outcome and self-reported health status after surgery for degenerative cervical myelopathy. Spine J 19(11):1809–1815. https://doi.org/10.1016/j.spinee.2019.06.026
Zika J, Alexiou GA, Giannopoulos S, Kastanioudakis I, Kyritsis AP, Voulgaris S (2020) Outcome factors in surgically treated patients for cervical spondylotic myelopathy. J Spinal Cord Med 43(2):206–210. https://doi.org/10.1080/10790268.2018.1500225
Kato S, Oshima Y, Oka H et al (2015) Comparison of the Japanese Orthopaedic Association (JOA) Score and Modified JOA (mJOA) Score for the Assessment of Cervical Myelopathy: A Multicenter Observational Study. PLoS One 10(4):e0123022. https://doi.org/10.1371/journal.pone.0123022
Wen Q, Kelley DA, Banerjee S et al (2015) Clinically feasible NODDI characterization of glioma using multiband EPI at 7 T. Neuroimage Clin 9:291–299. https://doi.org/10.1016/j.nicl.2015.08.017
Maximov II, Tonoyan AS, Pronin IN (2017) Differentiation of glioma malignancy grade using diffusion MRI. Phys Med 40:24–32. https://doi.org/10.1016/j.ejmp.2017.07.002
Acknowledgements
We thank Guangqi Li from the Center for Biomedical Imaging Research in Tsinghua University (Beijing, China) for his contribution to the figures and response for revision.
Funding
This study has received funding by the National Key Research and Development Program of China (2017YFC0108700), the National Natural Science Foundation of China (61571258), the National Natural Science Foundation of China (11871459), Tsinghua University Initiative Scientific Research Program (20161080166), Capital’s Funds for Health Improvement and Research (CFH2020-2–1121), Beijing JST Research Funding (ZR-201912), and Beijing Jishuitan Hospital Elite Young Scholar Programme (XKGG202103).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Huijun Chen (corresponding author).
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in
[10] Ma X, Han X, Jiang W, et al A Follow-up Study of Postoperative DCM Patients Using Diffusion MRI with DTI and NODDI[J]. Spine, 2018:1.
[11] Jiang W, Han X, Guo H, et al Usefulness of conventional magnetic resonance imaging, diffusion tensor imaging and neurite orientation dispersion and density imaging in evaluating postoperative function in patients with cervical spondylotic myelopathy[J]. Journal of Orthopaedic Translation, 2018, 15:59–69.
Methodology
• retrospective
• experimental
• performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chunyao Wang and Xiao Han are co-first authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, C., Han, X., Ma, X. et al. Spinal cord perfusion is associated with microstructural damage in cervical spondylotic myelopathy patients who underwent cervical laminoplasty. Eur Radiol 34, 1349–1357 (2024). https://doi.org/10.1007/s00330-023-10011-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-10011-9