Abstract
Objectives
To evaluate the ability of iodine uptake parameters from hepatic multiphasic CT to predict liver fibrosis, and compare absolute contrast enhancement (ΔHU) with dual-energy iodine density (ID) methods.
Methods
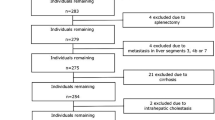
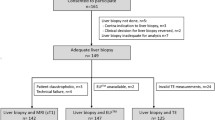
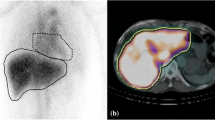
One hundred seventeen patients with pathologically proven liver fibrosis who underwent dual-energy CT during portal-venous phase (PVP) and 3-min delayed phase (DP) between January 2017 and Octotber 2019 were retrospectively included. Two radiologists measured the hepatic and blood-pool iodine uptake using ΔHU and ID methods; extracellular volume fraction (ECV) and the iodine washout rate (IWR) calculated with both methods were compared between different fibrosis stages (F0–1 vs. F2–4, F0–2 vs. F3–4, or F0–3 vs. F4). The inter-observer reproducibility (intraclass correlation coefficients [ICCs]) for ECV and IWR was compared between the ΔHU and ID methods. The areas under the receiver operating characteristic curves (AUCs) to predict liver fibrosis severity were calculated for serum and imaging fibrosis markers. To identify independent predictors, multivariable logistic regression analysis was performed, and combined performance was assessed for the ΔHU and ID models.
Results
Patients with F ≥ 2 (n = 70), F ≥ 3 (n = 51), and F4 (n = 29) had higher ECV and lower IWR than those with F ≤ 1, F ≤ 2, and F ≤ 3, respectively (all p < 0.001). ICCs were higher in the ID method than in the ΔHU method (ECV: p = 0.045; IWR: p < 0.001). The AUC ranges of ECVΔHU, ECVID, IWRΔHU, and IWRID for predicting liver fibrosis severity were 0.65–0.71, 0.67–0.73, 0.76–0.81, and 0.81–0.85, respectively. IWR and fibrosis-4 index were independent predictors, with combined AUCs of 0.82–0.87 for the ΔHU model and 0.86–0.89 for the ID model.
Conclusions
IWR more accurately predicted liver fibrosis than ECV in routine multiphasic CT. The dual-energy ID method yielded higher inter-observer reproducibility and predictive values than the single-energy ΔHU method.
Key Points
• The IWR calculated from hepatic iodine uptake during PVP and 3-min DP predicted liver fibrosis (AUC, 0.76–0.85), while the ECV had a relatively limited predictive value (ACU, 0.65–0.73).
• Compared with the conventional ΔHU method, the dual-energy ID method provided superior inter-observer reproducibility for measurement of ECV (p = 0.046) and IWR (p < 0.001).
• The IWR and FIB-4 served as independent predictors of liver fibrosis; their combination yielded the high diagnostic performance particularly when using the ID method (combined AUCs of 0.86–0.89).






Similar content being viewed by others
Change history
11 May 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00330-021-07994-8
Abbreviations
- APRI:
-
Alanine aminotransferase-to-platelet ratio index
- DP:
-
Delayed phase
- ECV:
-
Extracellular volume fraction
- FIB-4:
-
Fibrosis-4 index
- ICC:
-
Intraclass correlation coefficient
- ID:
-
Iodine density
- IWR:
-
Iodine washout rate
- PVP:
-
Portal-venous phase
- ΔHU:
-
Absolute contrast enhancement
References
Heidelbaugh JJ, Bruderly M (2006) Cirrhosis and chronic liver failure: part I. Diagnosis and evaluation. Am Fam Physician 74:756–762
de Franchis R (2015) Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 63:743–752
Bedossa P (2008) Liver biopsy. Gastroenterol Clin Biol 32:4–7
Horowitz JM, Venkatesh SK, Ehman RL et al (2017) Evaluation of hepatic fibrosis: a review from the society of abdominal radiology disease focus panel. Abdom Radiol (NY) 42:2037–2053
Varenika V, Fu Y, Maher JJ et al (2013) Hepatic fibrosis: evaluation with semiquantitative contrast-enhanced CT. Radiology 266:151–158
Zissen MH, Wang ZJ, Yee J, Aslam R, Monto A, Yeh BM (2013) Contrast-enhanced CT quantification of the hepatic fractional extracellular space: correlation with diffuse liver disease severity. AJR Am J Roentgenol 201:1204–1210
Bandula S, Punwani S, Rosenberg WM et al (2015) Equilibrium contrast-enhanced CT imaging to evaluate hepatic fibrosis: initial validation by comparison with histopathologic sampling. Radiology 275:136–143
Yoon JH, Lee JM, Klotz E et al (2015) Estimation of hepatic extracellular volume fraction using multiphasic liver computed tomography for hepatic fibrosis grading. Invest Radiol 50:290–296
Guo SL, Su LN, Zhai YN et al (2017) The clinical value of hepatic extracellular volume fraction using routine multiphasic contrast-enhanced liver CT for staging liver fibrosis. Clin Radiol 72:242–246
Shinagawa Y, Sakamoto K, Sato K, Ito E, Urakawa H, Yoshimitsu K (2018) Usefulness of new subtraction algorithm in estimating degree of liver fibrosis by calculating extracellular volume fraction obtained from routine liver CT protocol equilibrium phase data: preliminary experience. Eur J Radiol 103:99–104
Ito E, Sato K, Yamamoto R, Sakamoto K, Urakawa H, Yoshimitsu K (2020) Usefulness of iodine-blood material density images in estimating degree of liver fibrosis by calculating extracellular volume fraction obtained from routine dual-energy liver CT protocol equilibrium phase data: preliminary experience. Jpn J Radiol. https://doi.org/10.1007/s11604-019-00918-z
Vignaux O, Legmann P, Coste J, Hoeffel C, Bonnin A (1999) Cirrhotic liver enhancement on dual-phase helical CT: comparison with noncirrhotic livers in 146 patients. AJR Am J Roentgenol 173:1193–1197
Guan S, Zhao WD, Zhou KR, Peng WJ, Mao J, Tang F (2005) CT perfusion at early stage of hepatic diffuse disease. World J Gastroenterol 11:3465–3467
Lv P, Lin X, Gao J, Chen K (2012) Spectral CT: preliminary studies in the liver cirrhosis. Korean J Radiol 13:434–442
Zhao LQ, He W, Yan B, Wang HY, Wang J (2013) The evaluation of haemodynamics in cirrhotic patients with spectral CT. Br J Radiol 86:20130228
Van Beers BE, Leconte I, Materne R, Smith AM, Jamart J, Horsmans Y (2001) Hepatic Perfusion Parameters in Chronic Liver Disease. AJR Am J Roentgenol 176:667–673
Ronot M, Asselah T, Paradis V et al (2010) Liver fibrosis in chronic hepatitis C virus infection: differentiating minimal from intermediate fibrosis with perfusion CT. Radiology 256:135–142
Lamb P, Sahani DV, Fuentes-Orrego JM, Patino M, Ghosh A, Mendonca PR (2015) Stratification of patients with liver fibrosis using dual-energy CT. IEEE Trans Med Imaging 34:807–815
Sofue K, Tsurusaki M, Mileto A et al (2018) Dual-energy computed tomography for non-invasive staging of liver fibrosis: accuracy of iodine density measurements from contrast-enhanced data. Hepatol Res 48:1008–1019
Nagayama Y, Tanoue S, Inoue T et al (2020) Dual-layer spectral CT improves image quality of multiphasic pancreas CT in patients with pancreatic ductal adenocarcinoma. Eur Radiol 30:394–403
Mileto A, Sofue K, Marin D (2016) Imaging the renal lesion with dual-energy multidetector CT and multi-energy applications in clinical practice: what can it truly do for you? Eur Radiol 26:3677–3690
Patino M, Prochowski A, Agrawal MD et al (2016) Material separation using dual-energy CT: current and emerging applications. Radiographics 36:1087–1105
Marin D, Davis D, Roy Choudhury K et al (2017) Characterization of small focal renal lesions: diagnostic accuracy with single-phase contrast-enhanced dual-energy CT with material attenuation analysis compared with conventional attenuation measurements. Radiology 284:737–747
Pourvaziri A, Parakh A, Mojtahed A, Kambadakone A, Sahani DV (2019) Diagnostic performance of dual-energy CT and subtraction CT for renal lesion detection and characterization. Eur Radiol 29:6559–6570
Nagayama Y, Inoue T, Oda S et al (2020) Adrenal Adenomas versus Metastases: Diagnostic Performance of Dual-Energy Spectral CT Virtual Noncontrast Imaging and Iodine Maps. Radiology 296:324–332
Wai CT, Greenson JK, Fontana RJ et al (2003) A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 38:518–526
Sterling RK, Lissen E, Clumeck N et al (2006) Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 43:1317–1325
Jablonowski R, Wilson MW, Do L, Hetts SW, Saeed M (2015) Multidetector CT measurement of myocardial extracellular volume in acute patchy and contiguous infarction: validation with microscopic measurement. Radiology 274:370–378
Kawel N, Nacif M, Zavodni A et al (2012) T1 mapping of the myocardium: intra-individual assessment of post-contrast T1 time evolution and extracellular volume fraction at 3T for Gd-DTPA and Gd-BOPTA. J Cardiovasc Magn Reson 14:26
Soyer P, Dufresne AC, Somveille E, Scherrer A (1997) MR imaging of the liver: effect of portal hypertension on hepatic parenchymal enhancement using a gadolinium chelate. J Magn Reson Imaging 7:142–146
Materne R, Van Beers BE, Smith AM et al (2000) Non-invasive quantification of liver perfusion with dynamic computed tomography and a dual-input one-compartmental model. Clin Sci (Lond) 99:517–525
Hagiwara M, Rusinek H, Lee VS et al (2008) Advanced liver fibrosis: diagnosis with 3D whole-liver perfusion MR imaging--initial experience. Radiology 246:926–934
Zhou L, Chen TW, Zhang XM et al (2014) Liver dynamic contrast-enhanced MRI for staging liver fibrosis in a piglet model. J Magn Reson Imaging 39:872–878
Son JH, Lee SS, Lee Y et al (2020) Assessment of liver fibrosis severity using computed tomography-based liver and spleen volumetric indices in patients with chronic liver disease. Eur Radiol 30:3486–3496
Emoto T, Oda S, Kidoh M et al (2020) Myocardial extracellular volume quantification using cardiac computed tomography: a comparison of the dual-energy iodine method and the standard subtraction method. Acad Radiol. https://doi.org/10.1016/j.acra.2020.03.019
Oda S, Emoto T, Nakaura T et al (2019) Myocardial Late Iodine Enhancement and Extracellular Volume Quantification with Dual-Layer Spectral Detector Dual-Energy Cardiac CT. Radiology: Cardiothoracic Imaging 1:e180003
Nagayama Y, Iyama A, Oda S et al (2019) Dual-layer dual-energy computed tomography for the assessment of hypovascular hepatic metastases: impact of closing k-edge on image quality and lesion detectability. Eur Radiol 29:2837–2847
Nagayama Y, Nakaura T, Oda S et al (2018) Dual-layer DECT for multiphasic hepatic CT with 50 percent iodine load: a matched-pair comparison with a 120 kVp protocol. Eur Radiol 28:1719–1730
Yoon JH, Chang W, Lee ES, Lee SM, Lee JM (2020) Double low-dose dual-energy liver CT in patients at high-risk of hcc: a prospective, randomized, single-center study. Invest Radiol 55:340-348
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Toshinori Hirai.
Conflict of interest
The authors of this manuscript declare no relationships with any companies.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the institutional review board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
-
retrospective
-
diagnostic or prognostic study
-
performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In Table 4, the values for ΔHUliver_DP (HU) in F0-2 and F0-3 groups were incorrect.
Supplementary Information
ESM 1
(DOCX 170 kb)
Rights and permissions
About this article
Cite this article
Nagayama, Y., Kato, Y., Inoue, T. et al. Liver fibrosis assessment with multiphasic dual-energy CT: diagnostic performance of iodine uptake parameters. Eur Radiol 31, 5779–5790 (2021). https://doi.org/10.1007/s00330-021-07706-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-07706-2