Abstract
Purpose
To compare the effect of dual-energy CT (DECT) material density datasets on diagnostic performance, readers’ confidence, and interpretation time for renal lesion detection and characterization in comparison to subtraction CT (SCT).
Material and methods
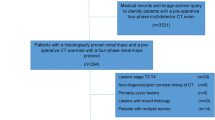
One hundred fourteen patients (69/45 = M/F, mean age = 67 years) who underwent contrast-enhanced DECT between January 2015 and February 2018 for suspected renal mass were included retrospectively. For each patient, three radiologists assessed three image datasets: group A, material density iodine (MDI) + material density water (MDW); group B, SCT only; and group C, SCT + true unenhanced phase + virtual monochromatic images at 65 keV. Readers evaluated image quality (4-point scale), the number of lesions, and likely diagnosis. Reading times were recorded. Quantitatively, iodine concentration (IC from MDI) and delta Hounsfield units (ΔHU) for all lesions were measured. Diagnostic accuracy was compared using the area under the receiver operating characteristic curve (AUC). Image quality and interpretation time were compared with Kruskal-Wallis and t tests.
Results
Study cohort (230 lesions; mean size = 23.63 mm (5–116 mm)) consisted of 60 enhancing, 158 non-enhancing, and 12 lipid-dominant angiomyolipoma lesions. Significantly higher image quality was demonstrated for MDI compared to SCT (mean score = 3.82 vs. 3; p < 0.05). Comparable diagnostic accuracy was observed for group A (AUC = 0.88) and group C (AUC = 0.87) and was higher compared to that for group B (AUC = 0.75). Group A was read faster than group C (41.49 s vs. 71.45 s per exam; p < 0.05). Both IC and ΔHU values had high accuracy (AUC = 0.97) for differentiating enhancing vs. non-enhancing lesions; however, IC enabled differentiation of clear cell renal cell carcinoma from other enhancing lesions with moderate accuracy (AUC = 0.73).
Conclusion
MDI images increase readers’ confidence for renal lesion detection and characterization while providing a more efficient radiologist workflow, irrespective of readers’ experience.
Key Points
• Material density iodine (MDI) images enable faster interpretation due to high image quality and potentially reduced need for quantitation.
• MDI images increase diagnostic confidence of readers, irrespective of radiologists’ experience.
• High accuracy with dual-energy CT (DECT) can potentially reduce healthcare costs by eliminating the need for additional investigations.






Similar content being viewed by others
Change history
11 July 2019
The original version of this article, published on 27 May 2019, unfortunately contained a mistake. The following correction has therefore been made in the original.
Abbreviations
- AUC:
-
Area under the receiver operating characteristic curve
- cRCC:
-
Clear cell renal cell carcinoma
- DECT:
-
Dual-energy CT
- dsDECT:
-
Dual-source dual-energy CT
- IC:
-
Iodine concentration
- IQ:
-
Image quality
- MDI:
-
Material density iodine
- MDW:
-
Material density water
- NIC:
-
Normalized iodine concentration
- R-CT:
-
Multiphasic renal mass protocol with conventional CT
- ROC:
-
Receiver operating characteristic
- ROI:
-
Region of interest
- rsDECT:
-
Rapid kV-switching dual-energy CT
- SCT:
-
Subtraction CT
- TS:
-
Tuberous sclerosis
- TUE:
-
True unenhanced
- PACS:
-
Picture archiving and communication system
- RCC:
-
Renal cell carcinoma
- SECT:
-
Stimulated single-energy CT
References
Al Harbi F, Tabatabaeefar L, Jewett MA, Finelli A, O’Malley M, Atri M (2016) Enhancement threshold of small (< 4 cm) solid renal masses on CT. AJR Am J Roentgenol 206:554–558
Apfaltrer P, Meyer M, Meier C et al (2012) Contrast-enhanced dual-energy CT of gastrointestinal stromal tumors: is iodine-related attenuation a potential indicator of tumor response? Invest Radiol 47:65
Ascenti G, Mileto A, Krauss B et al (2013) Distinguishing enhancing from nonenhancing renal masses with dual-source dual-energy CT: iodine quantification versus standard enhancement measurements. Eur Radiol 23:2288–2295
Baerends E, Oostveen LJ, Smit CT et al (2018) Comparing dual energy CT and subtraction CT on a phantom: which one provides the best contrast in iodine maps for sub-centimetre details? Eur Radiol 28:5051–5059
Birnbaum BA, Maki DD, Chakraborty DP, Jacobs JE, Babb JS (2002) Renal cyst pseudoenhancement: evaluation with an anthropomorphic body CT phantom. Radiology 225:83–90
Birnbaum BA, Hindman N, Lee J, Babb JS (2007) Multi–detector row CT attenuation measurements: assessment of intra- and interscanner variability with an anthropomorphic body CT phantom. Radiology 242:109–119
Borhani AA, Kulzer M, Iranpour N et al (2017) Comparison of true unenhanced and virtual unenhanced (VUE) attenuation values in abdominopelvic single-source rapid kilovoltage-switching spectral CT. Abdom Radiol (NY) 42:710–717
Cha D, Kim CK, Park JJ, Park BK (2016) Evaluation of hyperdense renal lesions incidentally detected on single-phase post-contrast CT using dual-energy CT. Br J Radiol 89:20150860
Chandarana H, Megibow AJ, Cohen BA et al (2011) Iodine quantification with dual-energy CT: phantom study and preliminary experience with renal masses. AJR Am J Roentgenol 196:W693–W700
Chandler A, Wei W, Herron DH, Anderson EF, Johnson VE, Ng CS (2011) Semiautomated motion correction of tumors in lung CT-perfusion studies. Acad Radiol 18:286–293
Chandler A, Wei W, Anderson EF, Herron DH, Ye Z, Ng CS (2012) Validation of motion correction techniques for liver CT perfusion studies. Br J Radiol 85:e514–e522
Dillman JR, Caoili EM, Cohan RH, Ellis JH, Francis IR, Schipper MJ (2008) Detection of upper tract urothelial neoplasms: sensitivity of axial, coronal reformatted, and curved-planar reformatted image-types utilizing 16-row multi-detector CT urography. Abdom Imaging 33:707–716
Ding A, Eisenberg JD, Pandharipande PV (2011) The economic burden of incidentally detected findings. Radiol Clin North Am 49:257–265
Eisner B, Kambadakone A, Samir A, Sahani D (2010) 619 subtraction ct improves radiologist confidence in evaluation of enhancing renal lesions. J Urol 183:e243–e244
Feuerlein S, Heye TJ, Bashir MR, Boll DT (2012) Iodine quantification using dual-energy multidetector computed tomography imaging: phantom study assessing the impact of iterative reconstruction schemes and patient habitus on accuracy. Invest Radiol 47:656
Goodsitt MM, Christodoulou EG, Larson SC (2011) Accuracies of the synthesized monochromatic CT numbers and effective atomic numbers obtained with a rapid kVp switching dual energy CT scanner. Med Phys 38:2222–2232
Graser A, Johnson TR, Hecht EM et al (2009) Dual-energy CT in patients suspected of having renal masses: can virtual nonenhanced images replace true nonenhanced images? Radiology 252:433–440
Graser A, Becker CR, Staehler M et al (2010) Single-phase dual-energy CT allows for characterization of renal masses as benign or malignant. Invest Radiol 45:399
Herts BR, Silverman SG, Hindman NM et al (2018) Management of the incidental renal mass on CT: a white paper of the ACR Incidental Findings Committee. J Am Coll Radiol 15:264–273
Hock LM, Lynch J, Balaji KC (2002) Increasing incidence of all stages of kidney cancer in the last 2 decades in the United States: an analysis of surveillance, epidemiology and end results program data. J Urol 167:57–60
Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK (2006) Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst 98:1331–1334
Jamis-Dow CA, Choyke PL, Jennings SB, Linehan WM, Thakore KN, Walther MM (1996) Small (< or = 3-cm) renal masses: detection with CT versus US and pathologic correlation. Radiology 198:785–788
Jayson M, Sanders H (1998) Increased incidence of serendipitously discovered renal cell carcinoma. Urology 51:203–205
Jinzaki M, Tanimoto A, Mukai M et al (2000) Double-phase helical CT of small renal parenchymal neoplasms: correlation with pathologic findings and tumor angiogenesis. J Comput Assist Tomogr 24:835
Kambadakone A, Arasu VA, Samir AE et al (2012) Qualitative assessment of enhancement in a renal mass: contribution of subtraction CT. J Comput Assist Tomogr 36:381
Kaza RK, Caoili EM, Cohan RH, Platt JF (2011) Distinguishing enhancing from nonenhancing renal lesions with fast kilovoltage-switching dual-energy CT. AJR Am J Roentgenol 197:1375–1381
Koonce JD, Vliegenthart R, Schoepf UJ et al (2014) Accuracy of dual-energy computed tomography for the measurement of iodine concentration using cardiac CT protocols: validation in a phantom model. Eur Radiol 24:512–518
Mahmood U, Horvat N, Horvat JV et al (2018) Rapid switching kVp dual energy CT: value of reconstructed dual energy CT images and organ dose assessment in multiphasic liver CT exams. Eur J Radiol 102:102–108
Manoharan D, Sharma S, Das CJ, Kumar R, Singh G, Kumar P (2018) Single-acquisition triple-bolus dual-energy CT protocol for comprehensive evaluation of renal masses: a single-center randomized noninferiority trial. AJR Am J Roentgenol 211:W1–W11
Marin D, Davis D, Roy Choudhury K et al (2017) Characterization of small focal renal lesions: diagnostic accuracy with single-phase contrast-enhanced dual-energy CT with material attenuation analysis compared with conventional attenuation measurements. Radiology 284:737–747
Matsumoto K, Jinzaki M, Tanami Y, Ueno A, Yamada M, Kuribayashi S (2011) Virtual monochromatic spectral imaging with fast kilovoltage switching: improved image quality as compared with that obtained with conventional 120-kVp CT. Radiology 259:257–262
McCarthy CJ, Baliyan V, Kordbacheh H, Sajjad Z, Sahani D, Kambadakone A (2016) Radiology of renal stone disease. Int J Surg 36:638–646
Mileto A, Nelson RC, Samei E et al (2014) Impact of dual-energy multi–detector row CT with virtual monochromatic imaging on renal cyst pseudoenhancement: in vitro and in vivo study. Radiology 272:767–776
Mileto A, Marin D, Ramirez-Giraldo et al (2014b) Accuracy of contrast-enhanced dual-energy MDCT for the assessment of iodine uptake in renal lesions. AJR Am J Roentgenol 202:W466–W474
Mohr B, Brink M, Oostveen LJ, Schuijf JD, Prokop M (2016) Lung iodine mapping by subtraction with image registration allowing for tissue sliding. Proc. SPIE 9784, Medical Imaging 2016: Image Processing, 978442
Patel BN, Bibbey A, Choudhury KR, Leder RA, Nelson RC, Marin D (2017) Characterization of small (< 4 cm) focal renal lesions: diagnostic accuracy of spectral analysis using single-phase contrast-enhanced dual-energy CT. AJR Am J Roentgenol 209:815–825
Pelgrim GJ, van Hamersvelt RW, Willemink MJ et al (2017) Accuracy of iodine quantification using dual energy CT in latest generation dual source and dual layer CT. Eur Radiol 27:3904–3912
Porter ME (2010) What is value in health care? N Engl J Med 363:2477–2481
Savci G, Yazici Z, Sahin N, Akgöz S, Tuncel E (2006) Value of chemical shift subtraction MRI in characterization of adrenal masses. AJR Am J Roentgenol 186:130–135
Siegel CL, Fisher AJ, Bennett HF (1999) Interobserver variability in determining enhancement of renal masses on helical CT. AJR Am J Roentgenol 172:1207–1212
Tada S, Yamagishi J, Kobayashi H, Hata Y, Kobari T (1983) The incidence of simple renal cyst by computed tomography. Clin Radiol 34:437–439
Vasudevan A, Davies RJ, Shannon BA, Cohen RJ (2006) Incidental renal tumours: the frequency of benign lesions and the role of preoperative core biopsy. BJU Int 97:946–949
Yoshioka K, Tanaka R, Takagi H et al (2016) Diagnostic accuracy of a modified subtraction coronary CT angiography method with short breath-holding time: a feasibility study. Br J Radiol 89:20160489
Yu L, Christner JA, Leng S, Wang J, Fletcher JG, McCollough CH (2011) Virtual monochromatic imaging in dual-source dual-energy CT: radiation dose and image quality. Med Phys 38:6371–6379
Zarzour JG, Milner D, Valentin R et al (2017) Quantitative iodine content threshold for discrimination of renal cell carcinomas using rapid kV-switching dual-energy CT. Abdom Radiol (NY) 42:727–734
Acknowledgements
This work was conducted with support from the Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University, and its affiliated academic healthcare centers, or the National Institutes of Health.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dushyant Vasudeo Sahani.
Conflict of interest
Dushyant Vasudeo Sahani, MD has the following disclosures which are not relevant to this work: research grant support from GE, Philips, and Bayer Healthcare; royalties (Elsevier); and being a consultant of Allena Pharmaceuticals, GE Healthcare. Other authors have no relevant disclosure. The data was handled by an investigator with no conflict of interest.
Statistics and biometry
Hang Lee, Ph.D. kindly provided the statistical advice for this manuscript. One of the authors has significant statistical expertise.
Informed consent
Written informed consent was waived by the institutional review board.
Ethical approval
Institutional review board approval was obtained.
Methodology
• Retrospective
• diagnostic study
• performed in one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: The names of Avinash Kambadakone and Dushyant Vasudeo Sahani were presented incorrectly.
Rights and permissions
About this article
Cite this article
Pourvaziri, A., Parakh, A., Mojtahed, A. et al. Diagnostic performance of dual-energy CT and subtraction CT for renal lesion detection and characterization. Eur Radiol 29, 6559–6570 (2019). https://doi.org/10.1007/s00330-019-06224-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06224-6