Abstract
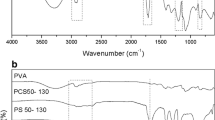
In this work, a comparative study of the swelling behavior of poly((1,7,7-trimethyl-2-bicyclo[2.2.1]heptanyl) prop-2-enoate) (poly(IBOA)) and its copolymer (poly(IBOA)-co-2-EHA)), prepared by radical polymerization with the addition of a low concentration of crosslinking agent and photoinitiator, was performed. The obtained networks were characterized by FTIR to show the conversion of acrylic band before and after polymerization and by differential scanning calorimetry. The copolymer network has a single glass transition temperature (Tg) that decreases relative to the poly(IBOA). The swelling study of poly(IBOA-co-2-EHA) was carried out by gravimetric method, in a series of polar (methanol, ethanol, propan-1-ol, butan-1-ol, pentan-1-ol, hexan-1-ol and heptan-1-ol) and non-polar (toluene) isotropic solvents. Solubility, interaction and diffusion parameters that influence the swelling of copolymer in the solvents were calculated. The well-established order of swelling (toluene > heptan-1-ol > hexan-1-ol > pentan-1-ol > butan-1-ol > propan-1-ol > ethanol > methanol) in the copolymer was clearly observed, and moreover, the degree of swelling increased with the presence of EHA in the copolymer. A simple diffusion model was applied (Fick's model) to interpret the swelling data. It was found that the nature of solvent shifts the mechanism from diffusion-controlled in the case of alcohols to non-Fickian one for toluene. For a long period, the experimental results were well correlated with the second-order diffusion kinetics of Schott.
Graphic abstract












Similar content being viewed by others
References
Wei C et al (2018) The kinetics of the polyacrylic superabsorbent polymers swelling in microalgae suspension to concentrate cells density. Bioresour Technol 249:713–719. https://doi.org/10.1016/j.biortech.2017.10.066
Zhang S-X et al (2017) Determination of the swelling behavior of superabsorbent polymers by a tracer-assisted on-line spectroscopic measurement. Polym Test 62:110–114. https://doi.org/10.1016/j.polymertesting.2017.06.020
Basta AH, Lotfy VF, Eldewany C (2021) Comparison of copper-crosslinked carboxymethyl cellulose versus biopolymer-based hydrogels for controlled release of fertilizer. Polym Plast Technol Mater 60(17):1884–1897. https://doi.org/10.1080/25740881.2021.1934017
Ni C, X.-X., Zhu, (2004) Synthesis and swelling behavior of thermosensitive hydrogels based on N-substituted acrylamides and sodium acrylate. Euro J Polym 40(6):1075–1080. https://doi.org/10.1016/j.eurpolymj.2003.12.017
Freeman B, Yampolskii Y, Pinnau I (2006) Materials science of membranes for gas and vapor separation. Wiley, New York
Vieth WR (1991) Diffusion in and through polymers: principles and applications. Hanser, München
Neogi P (1996) Diffusion in polymers. Marcel Dekkers Inc, New York
Zemzem M, Vinches L, Hallé S (2021) Molecular sorption and diffusion of organic solvents through maleated rubber/layered silicate nanocomposites. J Elastomers Plast. https://doi.org/10.1177/00952443211006162
Yin Y, Yang Y, Xu H (2002) Swelling behavior of hydrogels for colon-site drug delivery. J Appli Polym Sci 83(13):2835–2842. https://doi.org/10.1002/app.10259
Li Y et al (2022) Preparation of pH-responsive cellulose nanofibril/sodium alginate based hydrogels for drug release. J Appl Polym Sci 139(7):51647. https://doi.org/10.1002/app.51647
Liu M et al (2019) Ionic liquids as an effective additive for improving the solubility and rheological properties of hydrophobic associating polymers. J Mol Liquids 296:111833. https://doi.org/10.1016/j.molliq.2019.111833
Karadağ E et al (2021) Swelling equilibria of novel propenamide/2-acrylamido-2-methyl-1-propanesulfonic acid/guar gum/clinoptilolite biohybrid hydrogels and application as a sorbent for BV1 removal. Polym Bull 78(7):3625–3649. https://doi.org/10.1007/s00289-020-03285-2
Igarashi S et al (2006) Swelling signals of polymer films measured by a combination of micromechanical cantilever sensor and surface plasmon resonance spectroscopy. Sens Actuators B Chem 117(1):43–49. https://doi.org/10.1016/j.snb.2005.11.001
Tillman ES, Lewis NS (2003) Mechanism of enhanced sensitivity of linear poly(ethylenimine)-carbon black composite detectors to carboxylic acid vapors. Sens Actuators B Chem 96(1–2):329–342. https://doi.org/10.1016/S0925-4005(03)00567-7
Liu J, Zheng X, Tang K (2013) Study on the gravimetric measurement of the swelling behaviors of polymer films. Rev Adv Mater Sci 33(5):452–458
Lee JN, Park C, Whitesides GM (2003) Solvent compatibility of poly(dimethylsiloxane)-based microfluidic devices. Anal Chem 75(23):6544–6554. https://doi.org/10.1021/ac0346712
Cocchi G, De Angelis MG, Doghieri F (2015) Solubility and diffusivity of liquids for food and pharmaceutical applications in crosslinked polydimethylsiloxane (PDMS) films: I. Experimental data on pure organic components and vegetable oil. J Membr Sci 492:600–611. https://doi.org/10.1016/j.memsci.2015.04.063
Bedjaoui S et al (2020) Unusual swelling of acrylate based crosslinked polymer networks in linear primary alcohols: experimental and modeling aspects. J Mol Liquids 320:114459. https://doi.org/10.1016/j.molliq.2020.114459
Bye KP et al (2019) Pure and mixed fluid sorption and transport in Celazole® polybenzimidazole: effect of plasticization. J Membr Sci 580:235–247. https://doi.org/10.1016/j.memsci.2019.03.031
Odian G (1991) Principles of polymerization, 3rd edn. New York, Wiley
Bouchikhi N et al (2019) Photo-curing kinetics of hydroxyethyl acrylate (HEA): synergetic effect of dye/amine photoinitiator systems. Int J Ind Chem. https://doi.org/10.1007/s40090-019-00197-7
Peppas NA, Franson NM (1983) The swelling interface number as a criterion for prediction of diffusional solute release mechanisms in swellable polymers. J Polym Sci: Polym Phys Edition 21(6):983–997. https://doi.org/10.1002/pol.1983.180210614
Rudzinski W, Plazinski W (2009) On the applicability of the pseudo-second order equation to represent the kinetics of adsorption at solid/solution interfaces: a theoretical analysis based on the statistical rate theory. Adsorption 15(2):181–192. https://doi.org/10.1007/s10450-009-9167-8
Chatzi EG et al (1997) Infrared spectra and compositional analysis of styrene/2-ethylhexyl acrylate copolymers. Macromol Chem Phys 198(8):2409–2420. https://doi.org/10.1002/macp.1997.021980805
Boudraa K, Bouchaour T, Maschke U (2020) Thermal analysis of interpenetrating polymer networks through molecular dynamics simulations: a comparison with experiments. J Therm Anal Calorim 140(4):1845–1857. https://doi.org/10.1007/s10973-019-08898-y
Zeggai N et al (2018) Analysis of dynamic mechanical properties of photochemically crosslinked poly(isobornylacrylate-co-isobutylacrylate) applying WLF and Havriliak–-Negami models. Polym Test 72:432–438. https://doi.org/10.1016/j.polymertesting.2018.10.038
Shojaei AH, Paulson J, Honary S (2000) Evaluation of poly(acrylic acid-co-ethylhexyl acrylate) films for mucoadhesive transbuccal drug delivery: factors affecting the force of mucoadhesion. J Control Rel 67(2–3):223–232. https://doi.org/10.1016/S0168-3659(00)00216-9
Tanaka T (1986) Kinetics of phase transition in polymer gels. Physica A 140(1–2):261–268. https://doi.org/10.1016/0378-4371(86)90230-X
Tanaka T et al (1987) Mechanical instability of gels at the phase transition. Nature 325(6107):796–798. https://doi.org/10.1038/325796a0
Dervaux J, Amar MB (2012) Mechanical instabilities of gels. Annu Rev Condens Matter Phys 3(1):311–332. https://doi.org/10.1146/annurev-conmatphys-062910-140436
Burke J (1984) Solubility parameters: theory and application
Barton A (2013) CRC handbook of solubility parameters and other cohesion parameters. New York. https://doi.org/10.1201/9781315140575
Fedors RF (1974) A method for estimating both the solubility parameters and molar volumes of liquids. Polym Eng Sci 14(2):147–154. https://doi.org/10.1002/pen.760140211
Şen M, Güven O (1998) Determination of solubility parameter of poly(N-vinyl 2-pyrrolidon/ethylene glycol dimethacrylate) gels by swelling measurements. J Polym Sci Part B: Polym Phys 36(2):213–219. https://doi.org/10.1002/(SICI)10990488(19980130)36:2%3C213::AID-POLB2%3E3.0.CO;2-S
Hansen CM (2007) Hansen solubility parameters: a user’s handbook. CRC Press, Boca Raton. https://doi.org/10.1201/9781420006834
TrongáNguyen Q (1993) Sorption of organic solvents into dense silicone membranes. Part 1—validity and limitations of Flory–-Huggins and related theories. J Chem Soc Faraday Trans 89(24):4339–4346. https://doi.org/10.1039/FT9938904339
Ferrell WH, Kushner DI, Hickner MA (2017) Investigation of polymer–solvent interactions in poly(styrene sulfonate) thin films. J Polym Sci Part B: Polym Phys 55(18):1365–1372. https://doi.org/10.1002/polb.24383
Sudduth RD (2013) A review of the similarities and differences between five different polymer-solvent interaction coefficients. Pigment Resin Technol. https://doi.org/10.1108/PRT-07-2012-0042
Scott RL, Magat M (1949) Thermodynamics of high-polymer solutions. III. Swelling of cross-linked rubber. J Polym Sci 4(5):555–571. https://doi.org/10.1002/pol.1949.120040502
Blanks RF, Prausnitz J (1964) Thermodynamics of polymer solubility in polar and nonpolar systems. Ind Eng Chem Fundam 3(1):1–8. https://doi.org/10.1021/i160009a001
Bae Y et al (1993) Representation of vapor–liquid and liquid–liquid equilibria for binary systems containing polymers: applicability of an extended Flory–-Huggins equation. J Appl Polym Sci 47(7):1193–1206. https://doi.org/10.1002/app.1993.070470707
Favre E, Clément R, Nguyen QT, Schaetzel P, Néel J (1993) Sorption of organic solvents into dense silicone membranes. Part 2—Development of a new approach based on a clustering hypothesis for associated solvents. J Chem Soc Faraday Trans 89(24):4347–4353. https://doi.org/10.1039/FT9938904347
Kappert EJ et al (2019) Swelling of 9 polymers commonly employed for solvent-resistant nanofiltration membranes: a comprehensive dataset. J Membr Sci 569:177–199. https://doi.org/10.1016/j.memsci.2018.09.059
Chilin C, Metters A (2006) Hydrogels in controlled release formulations: network design and mathematical modelling. Adv Drug Deliv Rev 58:1379–1408. https://doi.org/10.1016/j.addr.2006.09.004
Wang C et al (2008) Synthesis and performance of novel hydrogels coatings for implantable glucose sensors. Biomacromol 9(2):561–567. https://doi.org/10.1021/bm701102y
Bajpai A, Bajpai J, Shukla S (2002) Water sorption through a semi-interpenetrating polymer network (IPN) with hydrophilic and hydrophobic chains. React Funct Polym 50(1):9–21. https://doi.org/10.1016/S1381-5148(01)00085-2
Crini G (2008) Kinetic and equilibrium studies on the removal of cationic dyes from aqueous solution by adsorption onto a cyclodextrin polymer. Dyes Pigm 77(2):415–426. https://doi.org/10.1016/j.dyepig.2007.07.001
Aminabhavi TM, Khinnavar RS (1993) Diffusion and sorption of organic liquids through polymer membranes: 10. Polyurethane, nitrile-butadiene rubber and epichlorohydrin versus aliphatic alcohols (C1–C5). Polymer 34(5):1006–1018. https://doi.org/10.1016/0032-3861(93)90222-V
Bernardo G (2013) Diffusivity of alcohols in amorphous polystyrene. J Appl Polym Sci 127(3):1803–1811. https://doi.org/10.1002/app.37918
Xu N, Xiao C (2011) Kinetics modeling and mechanism of organic matter absorption in functional fiber based on butyl methacrylate-hydroxyethyl methacrylate copolymer and low density polyethylene. Polym Plast Technol Eng 50(14):1496–1505. https://doi.org/10.1080/03602559.2011.593084
Acknowledgments
This report has been accomplished in the framework of an international research program. The authors gratefully acknowledge the support of the Algerian Ministry of Higher Education and Scientific Research (MESRS), the General Directorate of Scientific Research and Technological Development (DGRSDT) of Algeria, the University of Tlemcen in Algeria, the CNRS, and the University of Lille—Sciences and Technologies/France.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dounya, M., Maschke, U., Bouchikhi, N. et al. Characterization of swelling behavior and elastomer properties of acrylate polymers containing 2-ethylhexyl and isobornyl esters. Polym. Bull. 80, 10073–10098 (2023). https://doi.org/10.1007/s00289-022-04491-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04491-w