Abstract
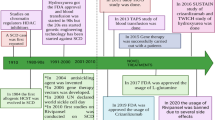
In 2022, sickle cell disease (SCD) continues to affect the lives of millions of people, being one of the most frequently inherited blood disorders worldwide. Recently, several new therapies have been FDA approved for the treatment of SCD. The complexity of the pathophysiology of sickling has given opportunity to the evolution of several modalities of therapies. Nonetheless, the potential for complementary targeting of HbS polymerization, vasocclusion, and other inflammatory pathways remains controversial. None of these drugs can be considered a single curative line of treatment. With the advancement of CRISPR/Cas9 technology, autologous transplant of gene-edited hematopoietic stem cells could possibly provide a cure for most patients with SCD. The advantage of this approach over the conventional stem cell transplantation is that it decreases the need for immuno-suppressive drugs and the risk of graft-versus-host disease. In addition, recent technological advances can reduce the off-target effects, but long-term monitoring is needed to ensure the reliability of these methods in the clinical setting. This review explores the efficacy and safety of combination therapies and contrasting this alternative with the challenges that exist with sickle cell gene therapy using CRISPR.


Similar content being viewed by others
References
DavidJ W (2010) The inherited diseases of hemoglobin are an emerging global health burden. Blood 115:4331–4336. https://doi.org/10.1182/blood-2010-01-251348
CDC (2020) Data & Statistics on Sickle Cell Disease | CDC. In: Cent. Dis. Control Prev. https://www.cdc.gov/ncbddd/sicklecell/data.html. Accessed 1 Nov 2021
Sedrak A, Kondamudi NP (2023) Sickle cell disease. In: StatPearls. StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK482384/
Pecker LH, Lanzkron S (2021) Sickle cell disease. Ann Intern Med 174:ITC1–ITC16. https://doi.org/10.7326/AITC202101190
Hilliard LM, Kulkarni V, Sen B et al (2018) Red blood cell transfusion therapy for sickle cell patients with frequent painful events. Pediatr Blood Cancer 65:e27423. https://doi.org/10.1002/pbc.27423
Adams RJ, McKie VC, Hsu L et al (1998) Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med 339:5–11. https://doi.org/10.1056/NEJM199807023390102
Scothorn DJ, Price C, Schwartz D et al (2002) Risk of recurrent stroke in children with sickle cell disease receiving blood transfusion therapy for at least five years after initial stroke. J Pediatr 140:348–354. https://doi.org/10.1067/mpd.2002.122498
Hulbert ML, McKinstry RC, Lacey JL et al (2011) Silent cerebral infarcts occur despite regular blood transfusion therapy after first strokes in children with sickle cell disease. Blood 117:772–779. https://doi.org/10.1182/blood-2010-01-261123
Ware RE, Helms RW (2012) Stroke with transfusions changing to hydroxyurea (SWiTCH). Blood 119:3925–3932. https://doi.org/10.1182/blood-2011-11-392340
Cokic VP, Smith RD, Beleslin-Cokic BB et al (2003) Hydroxyurea induces fetal hemoglobin by the nitric oxide–dependent activation of soluble guanylyl cyclase. J Clin Invest 111:231–239. https://doi.org/10.1172/JCI200316672
Agrawal RK, Patel RK, shah V, et al (2014) Hydroxyurea in sickle cell disease: drug review. Indian J Hematol Blood Transfus 30:91–96. https://doi.org/10.1007/s12288-013-0261-4
Niihara Y, Miller ST, Kanter J et al (2018) A phase 3 trial of L-glutamine in sickle cell disease. N Engl J Med 379:226–235. https://doi.org/10.1056/NEJMoa1715971
Dick MH, Abdelgadir A, Kulkarni VV et al (2022) Comparing the safety and efficacy of L-glutamine, voxelotor, and crizanlizumab for reducing the frequency of vaso-occlusive crisis in sickle cell disease: a systematic review. Cureus 14:e24920. https://doi.org/10.7759/cureus.24920
Ataga KI, Kutlar A, Kanter J et al (2017) Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med 376:429–439. https://doi.org/10.1056/NEJMoa1611770
Riley TR, Riley TT (2019) Profile of crizanlizumab and its potential in the prevention of pain crises in sickle cell disease: evidence to date. J Blood Med 10:307–311. https://doi.org/10.2147/JBM.S191423
Glaros AK, Razvi R, Shah N, Zaidi AU (2021) Voxelotor: alteration of sickle cell disease pathophysiology by a first-in-class polymerization inhibitor. Ther Adv Hematol 12:20406207211001136. https://doi.org/10.1177/20406207211001136
Shah N, Lipato T, Alvarez OA et al (2021) Real-world experience of voxelotor for the management of complications in sickle cell disease. Blood 138:2052. https://doi.org/10.1182/blood-2021-153138
Vichinsky E, Hoppe CC, Ataga KI et al (2019) A phase 3 randomized trial of voxelotor in sickle cell disease. N Engl J Med 381:509–519. https://doi.org/10.1056/NEJMoa1903212
Xu JZ, Conrey A, Frey I et al (2021) Mitapivat (AG-348) Demonstrates safety, tolerability, and improvements in anemia, hemolysis, oxygen affinity, and hemoglobin s polymerization kinetics in adults with sickle cell disease: a phase 1 dose escalation study. Blood 138:10. https://doi.org/10.1182/blood-2021-145398
Schroeder P, Fulzele K, Forsyth S et al (2022) Etavopivat, a pyruvate kinase activator in red blood cells, for the treatment of sickle cell disease. J Pharmacol Exp Ther 380:210–219. https://doi.org/10.1124/jpet.121.000743
van Dijk MJ, Rab MAE, van Oirschot BA et al (2022) Safety and efficacy of mitapivat, an oral pyruvate kinase activator, in sickle cell disease: a phase 2, open-label study. Am J Hematol 97:E226–E229. https://doi.org/10.1002/ajh.26554
Kalev-Zylinska ML, Hearn JI, Makhro A, Bogdanova A (2020) N-Methyl-D-aspartate receptors in hematopoietic cells: what have we learned? Front Physiol 11:577. https://doi.org/10.3389/fphys.2020.00577
Mahkro A, Hegemann I, Seiler E et al (2020) A pilot clinical phase II trial MemSID: acute and durable changes of red blood cells of sickle cell disease patients on memantine treatment. EJHaem 1:23–34. https://doi.org/10.1002/jha2.11
Tarasev M, Herppich A, Gao X, Hines P (2022) S107: P-Selectin inhibitor inclacumab reduces cell adhesion in an in-vitro assays showing potential for prevention of vaso-occlusion events in sickle cell disease. HemaSphere 6:3–4. https://doi.org/10.1097/01.HS9.0000821396.84589.71
Global Blood Therapeutics (2023) A randomized, double-blind, placebo-controlled, multicenter study to assess the safety and efficacy of inclacumab in participants with sickle cell disease experiencing vaso-occlusive crises. (Clinical Trial Registration NCT04935879). clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04935879
Assistance Publique - Hôpitaux de Paris (2022) Interest of famotidine in reducing endothelial expression of P-selectin in children with sickle cell disease: pilot study, single-center, prospective, non-comparative.(Clinical Trial Registration NCT05084521). clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT05084521
Lee MT, Ogu UO (2022) Sickle cell disease in the new era: advances in drug treatment. Transfus Apher Sci Off J World Apher Assoc Off J Eur Soc Haemapheresis 61:103555. https://doi.org/10.1016/j.transci.2022.103555
Salinas Cisneros G, Thein SL (2020) Recent advances in the treatment of sickle cell disease. Front Physiol 11:435. https://doi.org/10.3389/fphys.2020.00435
Ali MA, Ahmad A, Chaudry H et al (2020) Efficacy and safety of recently approved drugs for sickle cell disease: a review of clinical trials. Exp Hematol 92:11. https://doi.org/10.1016/j.exphem.2020.08.008
Hebbel RP, Hedlund BE (2018) Sickle hemoglobin oxygen affinity-shifting strategies have unequal cerebrovascular risks. Am J Hematol 93:321–325. https://doi.org/10.1002/ajh.24975
Pace BS, Starlard-Davenport A, Kutlar A (2021) Sickle cell disease: progress towards combination drug therapy. Br J Haematol 194:240–251. https://doi.org/10.1111/bjh.17312
Atweh GF, Sutton M, Nassif I et al (1999) Sustained induction of fetal hemoglobin by pulse butyrate therapy in sickle cell disease. Blood 93:1790–1797
Xu J, Peng C, Sankaran VG et al (2011) Correction of sickle cell disease in adult mice by interference with fetal hemoglobin silencing. Science 334:993–996. https://doi.org/10.1126/science.1211053
EMA (2023) Revocation of authorisation for sickle cell disease medicine Adakveo. In: Eur. Med. Agency. https://www.ema.europa.eu/en/news/revocation-authorisation-sickle-cell-disease-medicine-adakveo. Accessed 16 Sep 2023
Crizanlizumab versus placebo, with or without hydroxyurea/hydroxycarbamide, in adolescent and adult patients with sickle cell disease and vaso-occlusive crises: a randomized, double-blind, phase III study (STAND) | Blood | American Society of Hematology. https://ashpublications.org/blood/article/134/Supplement_1/998/427181/Crizanlizumab-Versus-Placebo-with-or-without. Accessed 16 Sep 2023
Johnson FL, Look AT, Gockerman J et al (1984) Bone-marrow transplantation in a patient with sickle-cell anemia. N Engl J Med 311:780–783. https://doi.org/10.1056/NEJM198409203111207
Bhalla N, Bhargav A, Yadav SK, Singh AK (2023) Allogeneic hematopoietic stem cell transplantation to cure sickle cell disease: a review. Front Med 10:1036939. https://doi.org/10.3389/fmed.2023.1036939
Bhatia M, Jin Z, Baker C et al (2014) Reduced toxicity, myeloablative conditioning with BU, fludarabine, alemtuzumab and SCT from sibling donors in children with sickle cell disease. Bone Marrow Transplant 49:913–920. https://doi.org/10.1038/bmt.2014.84
Hsieh MM, Fitzhugh CD, Weitzel RP et al (2014) Nonmyeloablative HLA-matched sibling allogeneic hematopoietic stem cell transplantation for severe sickle cell phenotype. JAMA 312:48–56. https://doi.org/10.1001/jama.2014.7192
Jacobsohn DA, Duerst R, Tse W, Kletzel M (2004) Reduced intensity haemopoietic stem-cell transplantation for treatment of non-malignant diseases in children. Lancet Lond Engl 364:156–162. https://doi.org/10.1016/S0140-6736(04)16628-2
Saraf SL, Oh AL, Patel PR et al (2016) Nonmyeloablative stem cell transplantation with alemtuzumab/low-dose irradiation to cure and improve the quality of life of adults with sickle cell disease. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant 22:441–448. https://doi.org/10.1016/j.bbmt.2015.08.036
Fitzhugh CD, Cordes S, Taylor T et al (2017) At least 20% donor myeloid chimerism is necessary to reverse the sickle phenotype after allogeneic HSCT. Blood 130:1946–1948. https://doi.org/10.1182/blood-2017-03-772392
Vermylen C, Cornu G, Ferster A et al (1998) Haematopoietic stem cell transplantation for sickle cell anaemia: the first 50 patients transplanted in Belgium. Bone Marrow Transplant 22:1–6. https://doi.org/10.1038/sj.bmt.1701291
Walters MC, Storb R, Patience M et al (2000) Impact of bone marrow transplantation for symptomatic sickle cell disease: an interim report. Multicenter investigation of bone marrow transplantation for sickle cell disease. Blood 95:1918–1924
Leonard A, Furstenau D, Abraham A et al (2023) Reduction in vaso-occlusive events following stem cell transplantation in patients with sickle cell disease. Blood Adv 7:227–234. https://doi.org/10.1182/bloodadvances.2022008137
Gaziev J, Isgrò A, Sodani P et al (2018) Haploidentical HSCT for hemoglobinopathies: improved outcomes with TCRαβ+/CD19+-depleted grafts. Blood Adv 2:263–270. https://doi.org/10.1182/bloodadvances.2017012005
Krishnamurti L (2021) Hematopoietic cell transplantation for sickle cell disease. Front Pediatr 8:551170. https://doi.org/10.3389/fped.2020.551170
Ophir E, Or-Geva N, Gurevich I et al (2013) Murine anti–third-party central-memory CD8+ T cells promote hematopoietic chimerism under mild conditioning: lymph-node sequestration and deletion of anti-donor T cells. Blood 121:1220–1228. https://doi.org/10.1182/blood-2012-07-441493
Singh AK, Schetzen E, Yadav SK et al (2021) Correction of murine sickle cell disease by allogeneic haematopoietic cell transplantation with anti-3rd party veto cells. Bone Marrow Transplant 56:1818–1827. https://doi.org/10.1038/s41409-021-01237-6
Frangoul H, Altshuler D, Cappellini MD et al (2021) CRISPR-Cas9 gene editing for sickle cell disease and β-thalassemia. N Engl J Med 384:252–260. https://doi.org/10.1056/NEJMoa2031054
Park SH, Bao G (2021) CRISPR/Cas9 gene editing for curing sickle cell disease. Transfus Apher Sci 60:103060. https://doi.org/10.1016/j.transci.2021.103060
Demirci S, Leonard A, Haro-Mora JJ et al (2019) CRISPR/Cas9 for sickle cell disease: applications, future possibilities, and challenges. In: Turksen K (ed) Cell biology and translational medicine, vol 5. stem cells: translational science to therapy. Springer International Publishing, Cham, pp 37–52
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors. Therefore, informed consent was not needed.
Conflict of interest
The authors declare no competing interests.
Disclaimer
All authors have participated in the formulation of this review and agree to its contents. This type of study “review article” includes and summarizes data originally published by other authors all referenced in this study, and it is not otherwise under active review by any other publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Youssry, I., Ayad, N. Sickle cell disease: combination new therapies vs. CRISPR-Cas9 potential and challenges — review article. Ann Hematol (2023). https://doi.org/10.1007/s00277-023-05510-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00277-023-05510-0