Abstract
Purpose
To compare various models of diffusion-weighted imaging including mono-exponential, bi-exponential, diffusion kurtosis (DK) and fractional-order calculus (FROC) models in diagnosing prostate cancer (PCa) in transition zone (TZ) and distinguish the high-grade PCa [Gleason score (GS) ≥ 7] lesions from the total of low-grade PCa (GS ≤ 6) lesions and benign prostatic hyperplasia (BPH) in TZ.
Methods
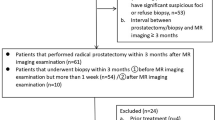
80 Patients with 103 lesions were included in this study. Nine metrics [including apparent diffusion coefficient (ADC) derived from mono-exponential model, slow diffusion coefficient (Ds), fast diffusion coefficient (Df),, and f (the fraction of fast diffusion) from bi-exponential model; mean diffusivity (MD) and mean kurtosis (MK) from DK model; diffusion coefficient (D), fractional-order derivative in space (β), and spatial metric (μ) from FROC model] were calculated. Comparisons between BPH and PCa lesions as well as between clinically significant PCa (CsPCa) (GS ≥ 7, n = 31) and clinically insignificant lesions (Cins) (GS ≤ 6 and BPH, n = 72) of these metrics were conducted. Mann–Whitney U-test and receiver operating characteristic (ROC) analysis were used for statistical evaluations.
Results
The areas under the ROC curve (AUC) values of β derived from FROC model were 0.778 and 0.853 in differentiating PCa from BPH and in differentiating CS (GS ≥ 7) from Cins (GS ≤ 6 and BPH), both were the highest compared to other metrics. The AUC value of β was significantly higher than that of ADC (P = 0.009) in differentiating CS from Cins, while the differentiation between BPH and PCa did not reach the statistical significance when comparing with ADC (P = 0.089).
Conclusion
Although no significant difference was found in distinguishing PCa from BPH, the metric β derived from FROC model was superior to other diffusion metrics in differentiation between CS and Cins in TZ.






Similar content being viewed by others
References
Culp MB, Soerjomataram I, Efstathiou JA, Bray F, Jemal A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur Urol 2020;77:38–52
Wilt TJ, MacDonald R, Rutks I, Shamliyan TA, Taylor BC, Kane RL. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med 2008;148:435–448
Bittencourt LK, Barentsz JO, de Miranda LC, Gasparetto EL. Prostate MRI: diffusion-weighted imaging at 1.5T correlates better with prostatectomy Gleason Grades than TRUS-guided biopsies in peripheral zone tumours. Eur Radiol 2012;22:468–475
Tamada T, Prabhu V, Li J, Babb JS, Taneja SS, Rosenkrantz AB. Prostate Cancer: Diffusion-weighted MR Imaging for Detection and Assessment of Aggressiveness-Comparison between Conventional and Kurtosis Models. Radiology 2017;284:100–108
deSouza NM, Riches SF, Vanas NJ, Morgan VA, Ashley SA, Fisher C, et al. Diffusion-weighted magnetic resonance imaging: a potential non-invasive marker of tumour aggressiveness in localized prostate cancer. Clin Radiol 2008;63:774–782
Turkbey B, Shah VP, Pang Y, Bernardo M, Xu S, Kruecker J, et al. Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images? Radiology 2011;258:488–495
Barbieri S, Bronnimann M, Boxler S, Vermathen P, Thoeny HC. Differentiation of prostate cancer lesions with high and with low Gleason score by diffusion-weighted MRI. Eur Radiol 2017;27:1547–1555
Itou Y, Nakanishi K, Narumi Y, Nishizawa Y, Tsukuma H. Clinical utility of apparent diffusion coefficient (ADC) values in patients with prostate cancer: can ADC values contribute to assess the aggressiveness of prostate cancer? J Magn Reson Imaging 2011;33:167–172
Woo S, Kim SY, Cho JY, Kim SH. Preoperative Evaluation of Prostate Cancer Aggressiveness: Using ADC and ADC Ratio in Determining Gleason Score. AJR Am J Roentgenol 2016;207:114–120
Bihan, D. Le, Robert Turner, and James R. Macfall. “Effects Of Intravoxel Incoherent Motions (Ivim) In Steady-State Free Precession (Ssfp) Imaging - Application To Molecular-Diffusion Imaging.” Magnetic Resonance in Medicine 1989;10: 324–337
Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: the quantification of non-Gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med 2005;53:1432–1440
Zhou XJ, Gao Q, Abdullah O, Magin RL. Studies of anomalous diffusion in the human brain using fractional order calculus. Magn Reson Med 2010; 63: 562–569.
Noda Y, Goshima S, Fujimoto K, Akamine Y, Kajita K, Kawai N, et al. Comparison of the Diagnostic Value of Mono-exponential, Bi-exponential, and Stretched Exponential Signal Models in Diffusion-weighted MR Imaging for Differentiating Benign and Malignant Hepatic Lesions. Magn Reson Med 2020.
Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988;168:497–505
Van Cauter S, Veraart J, Sijbers J, Peeters RR, Himmelreich U, De Keyzer F, et al. Gliomas: diffusion kurtosis MR imaging in grading. Radiology 2012;263:492–501
Liu X, Zhou L, Peng W, Wang C, Wang H. Differentiation of central gland prostate cancer from benign prostatic hyperplasia using mono-exponential and bi-exponential diffusion-weighted imaging. Magn Reson Imaging 2013;31:1318–1324
Ding K, Yao Y, Gao Y, Lu X, Chen H, Tang Q, et al. Diagnostic evaluation of diffusion kurtosis imaging for prostate cancer: Detection in a biopsy population. Eur J Radiol 2019;118:138–146
Si Y, Liu RB. Diagnostic Performance of Mono-exponential DWI Versus Diffusion Kurtosis Imaging in Prostate Cancer: A Systematic Review and Meta-Analysis. AJR Am J Roentgenol 2018;211:358–368
Zhang P, Min X, Wang L, Feng Z, Ke Z, You H, et al. Bi-exponential versus mono-exponential diffusion-weighted imaging for evaluating prostate cancer aggressiveness after radical prostatectomy: a whole-tumor histogram analysis. Acta Radiol 2019;60:1566–1575
Oto A, Kayhan A, Jiang Y, Tretiakova M, Yang C, Antic T, et al. Prostate cancer: differentiation of central gland cancer from benign prostatic hyperplasia by using diffusion-weighted and dynamic contrast-enhanced MR imaging. Radiology 2010;257:715–723
Noworolski SM, Vigneron DB, Chen AP, Kurhanewicz J. Dynamic contrast-enhanced MRI and MR diffusion imaging to distinguish between glandular and stromal prostatic tissues. Magn Reson Imaging 2008;26:1071–1080
Rosenkrantz AB, Kim S, Campbell N, Gaing B, Deng FM, Taneja SS. Transition zone prostate cancer: revisiting the role of multiparametric MRI at 3 T. AJR Am J Roentgenol 2015;204:W266–272.
Pierorazio PM, Walsh PC, Partin AW, Epstein JI. Prognostic Gleason grade grouping: data based on the modified Gleason scoring system. BJU Int 2013;111:753–760
Sorensen AG, Buonanno FS, Gonzalez RG, Schwamm LH, Lev MH, Huang-Hellinger FR, Reese TG, Weisskoff RM, Davis TL, Suwanwela N, Can U, Moreira JA, Copen WA, Look RB, Finklestein SP, Rosen BR, Koroshetz WJ. Hyperacute stroke: evaluation with combined multisection diffusion-weighted and hemodynamically weighted echo-planar MR imaging. Radiology 1996; 199:391–401
Tang L, Sui Y, Zhong Z, Damen FC, Li J, Shen L, et al. Non-Gaussian diffusion imaging with a fractional order calculus model to predict response of gastrointestinal stromal tumor to second-line sunitinib therapy. Magn Reson Med 2018;79:1399–1406
Tamura C, Shinmoto H, Soga S, Okamura T, Sato H, Okuaki T, et al. Diffusion Kurtosis Imaging Study of Prostate Cancer: Preliminary Findings. J Magn Reson Imaging 2014;40: 723–729
Rosenkrantz AB, Sigmund EE, Johnson G, Babb JS, Mussi TC, Melamed J, et al. Prostate cancer: feasibility and preliminary experience of a diffusional kurtosis model for detection and assessment of aggressiveness of peripheral zone cancer. Radiology 2012;264:126–135
Yang DM, Kim HC, Kim SW, Jahng GH, Won KY, Lim SJ, et al. Prostate cancer: correlation of intravoxel incoherent motion MR parameters with Gleason score. Clinical Imaging 2016;40: 445–450
Zhang M, Milot L, Khalvati F, Sugar L, Downes M, Baig SM, et al. Value of Increasing Biopsy Cores per Target with Cognitive MRI-targeted Transrectal US Prostate Biopsy. Radiology 2019;291:83–89
Sui Y, Wang H, Liu G, Damen FW, Wanamaker C, Li Y, et al. Differentiation of Low- and High-Grade Pediatric Brain Tumors with High b-Value Diffusion-weighted MR Imaging and a Fractional Order Calculus Model. Radiology 2015;277:489–496
Sui Y, Xiong Y, Jiang J, Karaman MM, Xie KL, Zhu W, et al. Differentiation of Low- and High-Grade Gliomas Using High b-Value Diffusion Imaging with a Non-Gaussian Diffusion Model. AJNR Am J Neuroradiol 2016;37:1643–1649
Gao Q, Srinivasan G, Magin RL, Zhou XJ. Anomalous diffusion measured by a twice-refocused spin echo pulse sequence: analysis using fractional order calculus. J Magn Reson Imaging 2011;33:1177–1183
Ingo C, Magin RL, Colon-Perez L, Triplett W, Mareci TH. On random walks and entropy in diffusion-weighted magnetic resonance imaging studies of neural tissue. Magn Reson Med 2014;71:617–627
Pang Y, Turkbey B, Bernardo M, Kruecker J, Kadoury S, Merino MJ, et al. Intravoxel incoherent motion MR imaging for prostate cancer: an evaluation of perfusion fraction and diffusion coefficient derived from different b-value combinations. Magn Reson Med 2013;69:553–562
Alonzi R, Padhani AR, Allen C. Dynamic contrast enhanced MRI in prostate cancer. Eur J Radiol 2007;63:335–350
Patel J, Sigmund EE, Rusinek H, Oei M, Babb JS, Taouli B. Diagnosis of cirrhosis with intravoxel incoherent motion diffusion MRI and dynamic contrast-enhanced MRI alone and in combination: preliminary experience. J Magn Reson Imaging 2010;31:589–600
Pesapane F, Patella F, Fumarola EM, Panella S, Ierardi AM, Pompili GG, et al. Intravoxel Incoherent Motion (IVIM) Diffusion Weighted Imaging (DWI) in the Periferic Prostate Cancer Detection and Stratification. Med Oncol 2017;34:35
Dennis LK, Lynch CF, Torner JC. Epidemiologic association between prostatitis and prostate cancer. Urology 2002;60:78–83
Zhang YD, Wang Q, Wu CJ, Wang XN, Zhang J, Liu H, et al. The histogram analysis of diffusion-weighted intravoxel incoherent motion (IVIM) imaging for differentiating the gleason grade of prostate cancer. Eur Radiol 2015;25:994–1004
Jensen JH, Helpern JA. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed 2010;23:698–710
Wang F, Chen HG, Zhang RY, Jin D, Xu SS, Wu GY, et al. Diffusion kurtosis imaging to assess correlations with clinicopathologic factors for bladder cancer: a comparison between the multi-b value method and the tensor method. Eur Radiol 2019;29:4447–4455
Mehralivand S, Bednarova S, Shih JH, Mertan FV, Gaur S, Merino MJ, et al. Prospective Evaluation of PI-RADS Version 2 Using the International Society of Urological Pathology Prostate Cancer Grade Group System. J Urol 2017;198:583–590
Funding
This study has received funding from Shanghai Shenkang Project (16CR3024A), Shanghai Jiaotong University Medical-Engineering Cross Fund (YG2017MS48) and Shanghai Science and Technology Committee of Shanghai Municipality (17441902700/18DZ1930104).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
We certify that each author has made substantive intellectual contributions to the paper and has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Liu, G., Lu, Y., Dai, Y. et al. Comparison of mono-exponential, bi-exponential, kurtosis, and fractional-order calculus models of diffusion-weighted imaging in characterizing prostate lesions in transition zone. Abdom Radiol 46, 2740–2750 (2021). https://doi.org/10.1007/s00261-020-02903-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-020-02903-x