Abstract
Purpose
To compare the diagnostic performance between diffusion kurtosis imaging (DKI) parameters and mono-exponential apparent diffusion coefficient (ADC) for determination of clinically significant cancer (CSC, Gleason score (GS) ≥ 7) in patients with histologically proven prostate cancer (PCa).
Methods
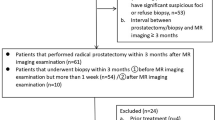
A total of 92 patients (mean age: 71.5 years, range: 47–89 years) who had been diagnosed as PCa and undergone 3 T-MRI including DWI (b values, 0, 100, 1000, 2000s/mm2) were included in this study. The DKI parameters, namely apparent diffusion for non-Gaussian distribution (Dapp) and apparent kurtosis coefficient (Kapp), were calculated by dedicated software using mono-exponential and diffusion kurtosis models for quantitation. The measurement was performed for a whole tumor after segmentation, and pathologic topographic maps or systemic biopsy results served as the reference standard for segmentation. To compare the diagnostic performance of each parameter for determination of CSC, pair-wise comparison of receiver operating characteristic (ROC) curves was performed.
Results
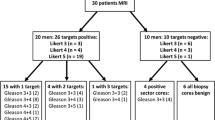
The study population consisted of GS 6 (n = 18), GS 7 (n = 31), GS 8 (n = 25), GS 9 (n = 15) and GS 10 (n = 3) patients. The area under the ROC curve of Kapp (0.707, 95% CI 0.603–0.798) for discriminating CSC from non-CSC was not significantly different from those of mono-exponential ADC (0.725, 0.622–0.813, P = 0.2175) or Dapp (0.726, 0.623–0.814, P = 0.9628). Diagnostic predictive values of Kapp were estimated to a maximum accuracy of 78%, a sensitivity of 86%, and a specificity of 47%, while those of mono-exponential ADC were 75, 81, and 53%, respectively.
Conclusion
The DKI parameters showed a diagnostic performance comparable to mono-exponential ADC for determination of CSC in patients with PCa.




Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018-2020) Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 68:394-424. https://doi.org/10.3322/caac.21492
Corcoran NM, Hong MK, Casey RG, Hurtado-Coll A, Peters J, Harewood L, Goldenberg SL, Hovens CM, Costello AJ, Gleave ME (2011) Upgrade in Gleason score between prostate biopsies and pathology following radical prostatectomy significantly impacts upon the risk of biochemical recurrence. BJU international 108 (8b):E202-E210. https://doi.org/https://doi.org/10.1111/j.1464-410X.2011.10119.x
Borghesi M, Ahmed H, Nam R, Schaeffer E, Schiavina R, Taneja S, Weidner W, Loeb S (2017) Complications after systematic, random, and image-guided prostate biopsy. European urology 71 (3):353-365. https://doi.org/https://doi.org/10.1016/j.eururo.2016.08.004
Vargas HA, Akin O, Franiel T, Mazaheri Y, Zheng J, Moskowitz C, Udo K, Eastham J, Hricak H (2011) Diffusion-weighted endorectal MR imaging at 3 T for prostate cancer: tumor detection and assessment of aggressiveness. Radiology 259 (3):775-784. https://doi.org/https://doi.org/10.1148/radiol.11102066
Jung SI, Donati OF, Vargas HA, Goldman D, Hricak H, Akin O (2013) Transition zone prostate cancer: incremental value of diffusion-weighted endorectal MR imaging in tumor detection and assessment of aggressiveness. Radiology 269 (2):493-503. https://doi.org/https://doi.org/10.1148/radiol.13130029
Si Y, Liu R-b (2018) Diagnostic performance of Monoexponential DWI versus diffusion kurtosis imaging in prostate Cancer: a systematic review and meta-analysis. American Journal of Roentgenology 211 (2):358-368. https://doi.org/https://doi.org/10.2214/AJR.17.18934
Rosenkrantz AB, Padhani AR, Chenevert TL, Koh DM, De Keyzer F, Taouli B, Le Bihan D (2015) Body diffusion kurtosis imaging: basic principles, applications, and considerations for clinical practice. Journal of Magnetic Resonance Imaging 42 (5):1190-1202. https://doi.org/https://doi.org/10.1002/jmri.24985
Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K (2005) Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 53 (6):1432-1440. https://doi.org/https://doi.org/10.1002/mrm.20508
Van Cauter S, Veraart J, Sijbers J, Peeters RR, Himmelreich U, De Keyzer F, Van Gool SW, Van Calenbergh F, De Vleeschouwer S, Van Hecke W (2012) Gliomas: diffusion kurtosis MR imaging in grading. Radiology 263 (2):492-501. https://doi.org/https://doi.org/10.1148/radiol.12110927
Barrett T, McLean M, Priest AN, Lawrence EM, Patterson AJ, Koo BC, Patterson I, Warren AY, Doble A, Gnanapragasam VJ (2018) Diagnostic evaluation of magnetization transfer and diffusion kurtosis imaging for prostate cancer detection in a re-biopsy population. European radiology 28 (8):3141-3150. https://doi.org/https://doi.org/10.1007/s00330-017-5169-1
Roethke MC, Kuder TA, Kuru TH, Fenchel M, Hadaschik BA, Laun FB, Schlemmer H-P, Stieltjes B (2015) Evaluation of diffusion kurtosis imaging versus standard diffusion imaging for detection and grading of peripheral zone prostate cancer. Investigative radiology 50 (8):483-489. https://doi.org/https://doi.org/10.1097/RLI.0000000000000155
Toivonen J, Merisaari H, Pesola M, Taimen P, Bostrom PJ, Pahikkala T, Aronen HJ, Jambor I (2015) Mathematical models for diffusion-weighted imaging of prostate cancer using b values up to 2000s/mm2: Correlation with Gleason score and repeatability of region of interest analysis. Magnetic resonance in medicine 74 (4):1116-1124. https://doi.org/https://doi.org/10.1002/mrm.25482
Wang Q, Li H, Yan X, Wu C-J, Liu X-S, Shi H-B, Zhang Y-D (2015) Histogram analysis of diffusion kurtosis magnetic resonance imaging in differentiation of pathologic Gleason grade of prostate cancer. In: Urologic oncology: seminars and original investigations. vol 8. Elsevier, pp 337. e315-337. e324. https://doi.org/https://doi.org/10.1016/j.urolonc.2015.05.005
Rosenkrantz AB, Sigmund EE, Johnson G, Babb JS, Mussi TC, Melamed J, Taneja SS, Lee VS, Jensen JH (2012) Prostate cancer: feasibility and preliminary experience of a diffusional kurtosis model for detection and assessment of aggressiveness of peripheral zone cancer. Radiology 264 (1):126-135. https://doi.org/https://doi.org/10.1148/radiol.12112290
Suo S, Chen X, Wu L, Zhang X, Yao Q, Fan Y, Wang H, Xu J (2014) Non-Gaussian water diffusion kurtosis imaging of prostate cancer. Magnetic resonance imaging 32 (5):421-427. https://doi.org/https://doi.org/10.1016/j.mri.2014.01.015
Wu C-J, Zhang Y-D, Bao M-L, Li H, Wang X-N, Liu X-S, Shi H-B (2017) Diffusion kurtosis imaging helps to predict upgrading in biopsy-proven prostate Cancer with a Gleason score of 6. American Journal of Roentgenology 209 (5):1081-1087. https://doi.org/https://doi.org/10.2214/AJR.16.17781
Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA (2016-2020) The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. The American journal of surgical pathology 40 (2):244-252. https://doi.org/10.1097/PAS.0000000000000530
Tamada T, Prabhu V, Li J, Babb JS, Taneja SS, Rosenkrantz AB (2017) Prostate cancer: diffusion-weighted MR imaging for detection and assessment of aggressiveness - comparison between conventional and kurtosis models. Radiology 284 (1):100-108. https://doi.org/https://doi.org/10.1148/radiol.2017162321
Shan Y, Chen X, Liu K, Zeng M, Zhou J (2019) Prostate cancer aggressive prediction: preponderant diagnostic performances of intravoxel incoherent motion (IVIM) imaging and diffusion kurtosis imaging (DKI) beyond ADC at 3.0 T scanner with gleason score at final pathology. Abdominal Radiology 44 (10):3441-3452. https://doi.org/https://doi.org/10.1007/s00261-019-02075-3
Ding K, Yao Y, Gao Y, Lu X, Chen H, Tang Q, Hua C, Zhou M, Zou X, Yin Q (2019) Diagnostic evaluation of diffusion kurtosis imaging for prostate cancer: Detection in a biopsy population. European journal of radiology 118:138-146. https://doi.org/https://doi.org/10.1016/j.ejrad.2019.07.009
Park SY, Jung DC, Oh YT, Cho NH, Choi YD, Rha KH, Hong SJ, Han K (2016) Prostate cancer: PI-RADS version 2 helps preoperatively predict clinically significant cancers. Radiology 280 (1):108-116. https://doi.org/https://doi.org/10.1148/radiol.16151133
Chen S, Yang Y, Peng T, Yu X, Deng H, Guo Z (2020) The prediction value of PI-RADS v2 score in high-grade Prostate Cancer: a multicenter retrospective study. International journal of medical sciences 17 (10):1366-1374. https://doi.org/https://doi.org/10.7150/ijms.45730
Epstein JI (2010) An update of the Gleason grading system. The Journal of urology 183 (2):433-440. https://doi.org/https://doi.org/10.1016/j.juro.2009.10.046
Bourne R, Kurniawan N, Cowin G, Sved P, Watson G (2011) 16 T diffusion microimaging of fixed prostate tissue: preliminary findings. Magnetic resonance in medicine 66 (1):244-247. https://doi.org/https://doi.org/10.1002/mrm.22778
Jia G, Abaza R, Williams JD, Zynger DL, Zhou J, Shah ZK, Patel M, Sammet S, Wei L, Bahnson RR (2011) Amide proton transfer MR imaging of prostate cancer: a preliminary study. Journal of Magnetic Resonance Imaging 33 (3):647-654. https://doi.org/https://doi.org/10.1002/jmri.22480
Takayama Y, Nishie A, Sugimoto M, Togao O, Asayama Y, Ishigami K, Ushijima Y, Okamoto D, Fujita N, Yokomizo A, Keupp J, Honda H (2016) Amide proton transfer (APT) magnetic resonance imaging of prostate cancer: comparison with Gleason scores. Magnetic Resonance Materials in Physics 29 (4):671-679. https://doi.org/https://doi.org/10.1007/s10334-016-0537-4
Jensen JH, Helpern JA (2010) MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR in Biomedicine 23 (7):698-710. https://doi.org/https://doi.org/10.1002/nbm.1518
Sun K, Chen X, Chai W, Fei X, Fu C, Yan X, Zhan Y, Chen K, Shen K, Yan F (2015) Breast cancer: diffusion kurtosis MR imaging - diagnostic accuracy and correlation with clinical-pathologic factors. Radiology 277 (1):46-55. https://doi.org/https://doi.org/10.1148/radiol.15141625
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This retrospective study was approved by the institutional review board, and informed consent was waived. (Approval Protocol Number: HPIRB 2020–01-001).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Park, H., Kim, S.H., Lee, Y. et al. Comparison of diagnostic performance between diffusion kurtosis imaging parameters and mono-exponential ADC for determination of clinically significant cancer in patients with prostate cancer. Abdom Radiol 45, 4235–4243 (2020). https://doi.org/10.1007/s00261-020-02776-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-020-02776-0