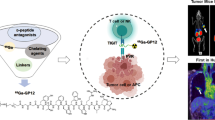
Abstract
Purpose
PD-L1 PET imaging allows for the whole body measuring its expression across primary and metastatic tumors and visualizing its spatiotemporal dynamics before, during, and after treatment. In this study, we reported a novel 18F-labeled D-peptide antagonist, 18F-NOTA-NF12, for PET imaging of PD-L1 status in preclinical and first-in-human studies.
Methods
Manual and automatic radiosynthesis of 18F-NOTA-NF12 was performed. Cell uptake and binding assays were completed in MC38, H1975, and A549 cell lines. The capacity for imaging of PD-L1 status, biodistribution, and pharmacokinetics were investigated in preclinical models. The PD-L1 status was verified by western blotting, immunohistochemistry/fluorescence, and flow cytometry. The safety, radiation dosimetry, biodistribution, and PD-L1 imaging potential were evaluated in healthy volunteers and patients.
Results
The radiosynthesis of 18F-NOTA-NF12 was achieved via manual and automatic methods with radiochemical yields of 41.7 ± 10.2 % and 70.6 ± 4.2 %, respectively. In vitro binding assays demonstrated high specificity and affinity with an IC50 of 78.35 nM and KD of 85.08 nM. The MC38 and H1975 tumors were clearly visualized with the optimized tumor-to-muscle ratios of 5.36 ± 1.17 and 7.13 ± 1.78 at 60 min after injection. Gemcitabine- and selumetinib-induced modulation of PD-L1 dynamics was monitored by 18F-NOTA-NF12. The tumor uptake correlated well with their PD-L1 expression. 18F-NOTA-NF12 exhibited renal excretion and rapid clearance from blood and other non-specific organs, contributing to high contrast imaging in the clinical time frame. In NSCLC and esophageal cancer patients, the specificity of 18F-NOTA-NF12 for PD-L1 imaging was confirmed. The 18F-NOTA-NF12 PET/CT and 18F-FDG PET/CT had equivalent findings in patients with high PD-L1 expression.
Conclusion
18F-NOTA-NF12 was developed successfully as a PD-L1-specific tracer with promising results in preclinical and first-in-human trials, which support the further validation of 18F-NOTA-NF12 for PET imaging of PD-L1 status in clinical settings.
Graphical abstract









Similar content being viewed by others
References
Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61.
Sharma P, Allison JP. Dissecting the mechanisms of immune checkpoint therapy. Nat Rev Immunol. 2020;20:75–6.
Xu W, Atkins MB, McDermott DF. Checkpoint inhibitor immunotherapy in kidney cancer. Nat Rev Urol. 2020;17:137–50.
Meric-Bernstam F, Larkin J, Tabernero J, Bonini C. Enhancing anti-tumour efficacy with immunotherapy combinations. Lancet. 2021;397:1010–22.
de Miguel M, Calvo E. Clinical challenges of immune checkpoint inhibitors. Cancer Cell. 2020;38:326–33.
Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–87.
Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17:542–51.
Lütje S, Feldmann G, Essler M, Brossart P, Bundschuh RA. Immune checkpoint imaging in oncology: a game changer toward personalized immunotherapy? J Nucl Med. 2020;61:1137–44.
van de Donk PP, Kist de Ruijter L, Lub-de Hooge MN, Brouwers AH, van der Wekken AJ, Oosting SF, et al. Molecular imaging biomarkers for immune checkpoint inhibitor therapy. Theranostics. 2020;10:1708–18.
Wei W, Rosenkrans ZT, Liu J, Huang G, Luo QY, Cai W. ImmunoPET: concept, design, and applications. Chem Rev. 2020;120:3787–851.
Bensch F, van der Veen EL, Lub-de Hooge MN, Jorritsma-Smit A, Boellaard R, Kok IC, et al. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat Med. 2018;24:1852–8.
Christensen C, Kristensen LK, Alfsen MZ, Nielsen CH, Kjaer A. Quantitative PET imaging of PD-L1 expression in xenograft and syngeneic tumour models using a site-specifically labelled PD-L1 antibody. Eur J Nucl Med Mol Imaging. 2020;47:1302–13.
Smit J, Borm FJ, Niemeijer AN, Huisman MC, Hoekstra OS, Boellaard R, et al. PD-L1 PET/CT imaging with radiolabeled durvalumab in patients with advanced stage non-small cell lung cancer. J Nucl Med. 2021. https://doi.org/10.2967/jnumed.121.262473.
Jung KH, Park JW, Lee JH, Moon SH, Cho YS, Lee KH. 89Zr-labeled anti-PD-L1 antibody PET monitors gemcitabine therapy-induced modulation of tumor PD-L1 expression. J Nucl Med. 2021;62:656–64.
Lv G, Sun X, Qiu L, Sun Y, Li K, Liu Q, et al. PET imaging of tumor PD-L1 expression with a highly specific nonblocking single-domain antibody. J Nucl Med. 2020;61:117–22.
Gao H, Wu Y, Shi J, Zhang X, Liu T, Hu B, et al. Nuclear imaging-guided PD-L1 blockade therapy increases effectiveness of cancer immunotherapy. J Immunother Cancer. 2020;8: e001156.
Xing Y, Chand G, Liu C, Cook GJR, O’Doherty J, Zhao L, et al. Early phase I study of a 99mTc-labeled anti-programmed death ligand-1 (PD-L1) single-domain antibody in SPECT/CT assessment of PD-L1 expression in non-small cell lung cancer. J Nucl Med. 2019;60:1213–20.
Donnelly DJ, Smith RA, Morin P, Lipovšek D, Gokemeijer J, Cohen D, et al. Synthesis and biologic evaluation of a novel 18F-labeled adnectin as a PET radioligand for imaging PD-L1 expression. J Nucl Med. 2018;59:529–35.
Huisman MC, Niemeijer AN, Windhorst AD, Schuit RC, Leung D, Hayes W, et al. Quantification of PD-L1 expression with 18F-BMS-986192 PET/CT in patients with advanced-stage non-small cell lung cancer. J Nucl Med. 2020;61:1455–60.
Nienhuis PH, Antunes IF, Glaudemans A, Jalving M, Leung D, Noordzij W, et al. 18F-BMS986192 PET imaging of PD-L1 in metastatic melanoma patients with brain metastases treated with immune checkpoint inhibitors: a pilot study. J Nucl Med. 2021. https://doi.org/10.2967/jnumed.121.262368.
De Silva RA, Kumar D, Lisok A, Chatterjee S, Wharram B, Venkateswara Rao K, et al. Peptide-based 68Ga-PET radiotracer for imaging PD-L1 expression in cancer. Mol Pharm. 2018;15:3946–52.
Kumar D, Mishra A, Lisok A, Kureshi R, Shelake S, Plyku D, et al. Pharmacodynamic measures within tumors expose differential activity of PD(L)-1 antibody therapeutics. Proc Natl Acad Sci USA. 2021;118: e2107982118.
Zhou X, Jiang J, Yang X, Liu T, Ding J, Nimmagadda S, et al. First-in-human evaluation of a PD-L1-binding peptide radiotracer in non-small cell lung cancer patients with PET. J Nucl Med. 2022;63:536–42.
Schumacher TNM, Mayr LM, Minor DL, Milhollen MA, Burgess MW, Kim PS. Identification of D-peptide ligands through mirror-image phage display. Science. 1996;271:1854–7.
Chang HN, Liu BY, Qi YK, Zhou Y, Chen YP, Pan KM, et al. Blocking of the PD-1/PD-L1 interaction by a D-peptide antagonist for cancer immunotherapy. Angew Chem Int Ed Engl. 2015;54:11760–4.
Wang S, Zhou X, Xu X, Ding J, Liu S, Hou X, et al. Clinical translational evaluation of Al18F-NOTA-FAPI for fibroblast activation protein-targeted tumour imaging. Eur J Nucl Med Mol Imaging. 2021;48:4259–71.
Liu Z, Yu L, Cheng K, Feng Y, Qiu P, Gai Y, et al. Optimization, automation and validation of the large-scale radiosynthesis of Al18F tracers in a custom-made automatic platform for high yield. React Chem Eng. 2020;5:1441–9.
Delbeke D, Coleman RE, Guiberteau MJ, et al. Procedure guideline for tumor imaging with 18F-FDG PET/CT 1.0. J Nucl Med. 2006;47:885–95.
Galluzzi L, Humeau J, Buqué A, Zitvogel L, Kroemer G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol. 2020;17:725–41.
Parsa AT, Waldron JS, Panner A, et al. Loss of tumor suppressor PTEN function increases B7–H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–8.
Stutvoet TS, van der Veen EL, Kol A, Antunes IF, de Vries EFJ, Hospers GAP, et al. Molecular imaging of PD-L1 expression and dynamics with the Adnectin-based PET tracer 18F-BMS-986192. J Nucl Med. 2020;61:1839–44.
Scott DE, Bayly AR, Abell C, Skidmore J. Small molecules, big targets: drug discovery faces the protein-protein interaction challenge. Nat Rev Drug Discov. 2016;15:533–50.
Niemeijer AN, Oprea Lager DE, Huisman MC, Hoekstra OS, Boellaard R, van de Veen B, et al. Study of 89Zr-pembrolizumab PET/CT in patients with advanced stage non-small-cell lung cancer. J Nucl Med. 2022;63:362–7.
Acknowledgements
The authors would like to acknowledge Dr. Zhiguo Liu (Shandong Cancer Hospital) for the design, assembly, and optimization of the ChelationLab@Al18F automatic platform, Dr. Chao Chen (Xiangya Hospital) for flow cytometry analysis, and Dr. Xin Yan (Sun Yat-sen University) for molecular docking.
Funding
This study was financially supported by the National Natural Science Foundation of China (91859207, 81801762, 81771873, and 91959122), the Joint Fund of National Natural Science Foundation of China - China National Nuclear Corporation for Nuclear Technology Innovation (U1967222), and the Natural Science Foundation of Hunan Province of China (2020JJ5956).
Author information
Authors and Affiliations
Contributions
Design and supervision: SH and XZ; methodology: XW, MZ, and BC; data collection and analysis: XW, MZ, BC, SX, WR, XY, ZZ, HL, and JF; patient recruitment, PET scan, and analysis: SH, PD, MZ, XW, and BC; manuscript writing, review, and editing: XW, SH, and XZ.
Corresponding authors
Ethics declarations
Ethics approval
All clinical studies were approved by the Medical Ethics Committee of Xiangya Hospital, Central South University (No. 202106115). All animal studies were performed according to the guidelines of the Animal Care Committee of Central South University.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Preclinical Imaging
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, M., Wang, X., Chen, B. et al. Preclinical and first-in-human evaluation of 18F-labeled D-peptide antagonist for PD-L1 status imaging with PET. Eur J Nucl Med Mol Imaging 49, 4312–4324 (2022). https://doi.org/10.1007/s00259-022-05876-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-05876-9