Abstract
Purpose
A same-day PET imaging agent capable of measuring PD-L1 status in tumors is an important tool for optimizing PD-1 and PD-L1 treatments. Herein we describe the discovery and evaluation of a novel, fluorine-18 labeled macrocyclic peptide-based PET ligand for imaging PD-L1.
Methods
[18F]BMS-986229 was synthesized via copper mediated click-chemistry to yield a PD-L1 PET ligand with picomolar affinity and was tested as an in-vivo tool for assessing PD-L1 expression.
Results
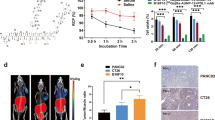
Autoradiography showed an 8:1 binding ratio in L2987 (PD-L1 (+)) vs. HT-29 (PD-L1 (-)) tumor tissues, with >90% specific binding. Specific radioligand binding (>90%) was observed in human non-small-cell lung cancer (NSCLC) and cynomolgus monkey spleen tissues. Images of PD-L1 (+) tissues in primates were characterized by high signal-to-noise, with low background signal in non-expressing tissues. PET imaging enabled clear visualization of PD-L1 expression in a murine model in vivo, with 5-fold higher uptake in L2987 (PD-L1 (+)) than in control HT-29 (PD-L1 (-)) tumors. Moreover, this imaging agent was used to measure target engagement of PD-L1 inhibitors (peptide or mAb), in PD-L1 (+) tumors as high as 97%.
Conclusion
A novel 18F-labeled macrocyclic peptide radioligand was developed for PET imaging of PD-L1 expressing tissues that demonstrated several advantages within a nonhuman primate model when compared directly to adnectin- or mAb-based ligands. Clinical studies are currently evaluating [18F]BMS-986229 to measure PD-L1 expression in tumors.







Similar content being viewed by others
References
Yu Xin J, Hubbard-Lucey VM, Tang J. Immuno-oncology drug development goes global. Nat Rev Drug Discov. 2019;18:899–900.
Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, Felip E, Broderick SR, Brahmer JR, Swanson SJ, Kerr K, Wang C, Ciuleanu T-E, Saylors GB, Tanaka F, Ito H, Chen K-N, Liberman M, Vokes EE, Taube JM, Dorange C, Cai J, Fiore J, Jarkowski A, Balli D, Sausen M, Pandya D, Calvet CY, Girard N. Neoadjuvant Nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973–85.
Riella LV, Paterson AM, Sharpe AH, Chandraker A. Role of the PD-1 pathway in the immune response. Am J Transplant. 2012;12:2575–87.
Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704.
Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–34.
Herbst RS, Soria J-C, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HEK, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature (London, U K). 2014;515:563–7.
Rittmeyer A, Barlesi F, Waterkamp D, Ballinger M, Kowanetz M, He P, Chen DS, Sandler A, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, Cortinovis DL, Leach J, Polikoff J, Barrios C, Kabbinavar F, Frontera OA, De MF, Turna H, Lee J-S, Gandara DR. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–65.
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim Y-C, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeño J, Wadsworth C, Melillo G, Jiang H, Huang Y, Dennis PA, Ozguroglu M. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919–29.
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–20.
Kazandjian D, Suzman DL, Blumenthal G, Mushti S, He K, Libeg M, Keegan P, Pazdur R. FDA approval summary: nivolumab for the treatment of metastatic non-small cell lung cancer with progression on or after platinum-based chemotherapy. Oncologist. 2016;21:634–42.
He J, Hu Y, Hu M, Li B. Development of PD-1/PD-L1 Pathway in Tumor Immune Microenvironment and Treatment for Non-Small Cell Lung Cancer. Sci Rep. 2015;5:13110.
Shukuya T, Carbone DP. Predictive Markers for the Efficacy of Anti-PD-1/PD-L1 Antibodies in Lung Cancer. J Thorac Oncol. 2016;11:976–88.
Robu S, Richter A, Seidl C, Weber WA, Gosmann D, Aulehner C, Krackhardt AM, Leung D, Hayes W, Cohen D, Morin P, Donnelly DJ, Lipovsek D, Bonacorsi SJ, Smith A, Steiger K, Steiger K, Krackhardt AM, Weber WA, Weber WA. Synthesis and preclinical evaluation of a [68Ga]-labeled adnectin, [68Ga]-BMS-986192, as a PET agent for imaging PD-L1 expression. J Nucl Med. 2021;62:1228–34.
Donnelly DJ, Smith RA, Morin P, Lipovsek D, Gokemeijer J, Cohen D, Lafont V, Tran T, Cole EL, Wright M, Kim J, Pena A, Kukral D, Dischino DD, Chow P, Gan J, Adelakun O, Wang XT, Cao K, Leung D, Bonacorsi SJ Jr, Hayes W. Synthesis and biologic evaluation of a novel 18F-labeled adnectin as a PET radioligand for imaging PD-L1 expression. J Nucl Med. 2018;59:529–35.
Niemeijer AN, Leung D, Huisman MC, Bahce I, Hoekstra OS, van Dongen GAMS, Boellaard R, Du S, Hayes W, Smith R, Windhorst AD, Hendrikse NH, Poot A, Vugts DJ, Thunnissen E, Morin P, Lipovsek D, Donnelly DJ, Bonacorsi SJ, Velasquez LM, de Gruijl TD, Smit EF, de Langen AJ. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat Commun. 2018;9:1–5.
Stutvoet TS, van der Veen EL, Kol A, Antunes IF, de Vries EFJ, Hospers GAP, de Vries EGE, de Jong S, Lub-de Hooge MN. Molecular imaging of PD-L1 expression and dynamics with the adnectin-based PET tracer 18F-BMS-986192. J Nucl Med. 2020;61:1839–45.
Zhou H, Bao G, Wang Z, Zhang B, Li D, Chen L, Deng X, Yu B, Zhao J, Zhu X, Zhao J, Zhao J. PET imaging of an optimized anti-PD-L1 probe [68Ga]-NODAGA-BMS-986192 in immunocompetent mice and non-human primates. EJNMMI Res. 2022;12:35.
Nienhuis PH, Antunes IF, Glaudemans AWJM, Noordzij W, Slart RHJA, de Vries EFJ, Jalving M, Hospers GAP, Leung D, Slart RHJA. 18F-BMS-986192 PET imaging of PD-L1 in metastatic melanoma patients with brain metastases treated with immune checkpoint inhibitors: a pilot study. J Nucl Med. 2022;63:899–905.
Donnelly David J. PET imaging in drug discovery and development. In: Handbook of Radiopharmaceuticals, 2nd edn. 2020;703–725. https://doi.org/10.1002/9781119500575.ch22.
Scola P, et al. Discovery of BMS-986189, a Macrocyclic Peptide Inhibitor of PD-L1, manuscript submitted to ACS Med. Chem Lett. 2023.
Cole EL, Kim J, Donnelly DJ, Smith RA, Cohen D, Lafont V, Morin PE, Huang RYC, Chow PL, Hayes W, Bonacorsi S Jr. Radiosynthesis and preclinical PET evaluation of 89Zr-nivolumab (BMS-936558) in healthy non-human primates. Bioorg Med Chem. 2017;25:5407–14.
Wei W, Zhang D, Wang C, Zhang Y, An S, Chen Y, Huang G, Liu J. Annotating cd38 expression in multiple myeloma with [18F]F-Nb1053. Mol Pharm. 2022;19(10):3502–10.
Kumar D, Lisok A, Dahmane E, McCoy M, Shelake S, Chatterjee S, Allaj V, Sysa-Shah P, Wharram B, Lesniak WG, Tully E, Gabrielson E, Jaffee EM, Poirier JT, Rudin CM, Gobburu JV, Pomper MG, Nimmagadda S. Peptide-based PET quantifies target engagement of PD-L1 therapeutics. J Clin Invest. 2019;129:616–30.
Lesniak WG, Mease RC, Chatterjee S, Kumar D, Lisok A, Wharram B, Kalagadda VR, Emens LA, Pomper MG, Nimmagadda S. Development of [18F]FPy-WL12 as a PD-L1 specific PET imaging peptide. Mol Imaging. 2019;18:1–9.
Jouini N, Cardinale J, Mindt TL, Jouini N, Mindt TL, Jouini N, Cardinale J, Mindt TL. Evaluation of a radiolabeled macrocyclic peptide as potential PET imaging probe for PD-L1. ChemMedChem. 2022;17: e202200091.
De Silva RA, Kumar D, Lisok A, Chatterjee S, Wharram B, Venkateswara Rao K, Mease R, Dannals RF, Pomper MG, Nimmagadda S. Peptide-based 68Ga-PET radiotracer for imaging PD-L1 expression in cancer. Mol Pharm. 2018;15:3946–52.
Lin D, Wallace M, Allentoff AJ, Donnelly DJ, Gomes E, Voronin K, Gong S, Huang RYC, Kim H, Caceres-Cortes J, Bonacorsi S. Chemoselective methionine bioconjugation: Site-selective fluorine-18 labeling of proteins and peptides. Bioconjug Chem. 2020;31:1908–16.
Donnelly D. Methods and compositions for 18F-radiolabeling of biologics. Methods and compositions for 18f-radiolabeling of biologics - Google Patents. Princeton , NJ: BRISTOL -M YERS SQUIBB Publication Classification Company. 2022.
Donnelly D, Leung DK. Imaging methods using 18F-radiolabeled biologics. Imaging methods using 18f-radiolabeled biologics - Google Patents. Princeton , NJ: BRISTOL -M YERS SQUIBB Publication Classification Company. 2021.
Brahmer JR, Tykodi SS, Chow LQM, Hwu W-J, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of Anti–PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65.
Hotchkiss RS, Colston E, Yende S, Angus DC, Moldawer LL, Crouser ED, Martin GS, Coopersmith CM, Brakenridge S, Mayr FB, Park PK, Ye J, Catlett IM, Girgis IG, Grasela DM. Immune checkpoint inhibition in sepsis: a phase 1b randomized, placebo-controlled, single ascending dose study of antiprogrammed cell death-ligand 1 antibody (BMS-936559). Crit Care Med. 2019;47:632–42.
Gay CL, Bosch RJ, Ritz J, Hataye JM, Aga E, Tressler RL, Mason SW, Hwang CK, Grasela DM, Ray N, Cyktor JC, Coffin JM, Acosta EP, Koup RA, Mellors JW, Eron JJ. Clinical trial of the anti-PD-L1 antibody BMS-936559 in HIV-1 infected participants on suppressive antiretroviral therapy. J Infect Dis. 2017;215:1725–33.
Postow MA, Mauguen A, Frosina D, et al. Assessing PD-L1 without a biopsy and through PD-L1 PET imaging with [18F]BMS-986229. J Clin Oncol. 2022;40(11):Supplement. Abstract #2578.
Cytryn S, Lumish M, Paroder V, et al. Feasibility, safety, and biodistribution of [18F]BMS-986229 PET in patients with esophagogastric (EG) cancer. Ann Oncol. 2022;33(Suppl_4):S335, P-244.
Acknowledgements
The authors would like to thank Michael Miller, Louis Lombardo, Percy Carter, Virginie Lafont, Yawu Jing and Wendy Hayes for their efforts in support of the work generated in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Authors: David J. Donnelly, Joonyoung Kim, Tritin Tran, Paul M. Scola, Daniel Tenney, Adrienne Pena, Thomas Petrone, Yunhui Zhang, Kenneth M. Boy, Michael A. Poss, Erin L. Cole, Matthew G. Soars, Benjamin M. Johnson, Daniel Cohen, Daniel Batalla, Patrick L. Chow, Andrea Olga Shorts, Shuyan Du, Nicholas A. Meanwell and Samuel J. Bonacorsi, Jr. were employees and stockholders of Bristol Myers Squibb Co. at the time of manuscript preparation. Ethical approval: All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures involving animal studies were reviewed and approved by the Bristol Myers Squibb animal care/use committee and in adherence to 3Rs principles of animal research (AALAC) https://www.bms.com/about-us/sustainability/environment/product-stewardship.html. This article does not contain any studies with human participants performed by any of the authors. [18F]BMS-986229, [18F]BMS-986192, BMS-986189, BMS-936559 and [18F]BMT-187144 are the subject of granted patents and pending patent applications US7943743, US8383796, WO2017201111A1 and W02017210302A1. No other conflict of interest relevant to this article was reported.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sadly, Tritin Tran passed away during the preparation of this manuscript, his contributions to the field of PET ligand discovery are sorely missed.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Donnelly, D.J., Kim, J., Tran, T. et al. The discovery and evaluation of [18F]BMS-986229, a novel macrocyclic peptide PET radioligand for the measurement of PD-L1 expression and in-vivo PD-L1 target engagement. Eur J Nucl Med Mol Imaging 51, 978–990 (2024). https://doi.org/10.1007/s00259-023-06527-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06527-3