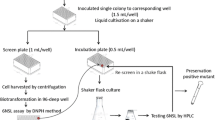
Abstract
Obtaining a sucrose isomerase (SIase) with high catalytic performance is of great importance in industrial production of isomaltulose (a reducing sugar). In order to obtain such SIase mutant, a high-throughput screening system in microtiter plate format was developed based on a widely used 2,4-dinitrosalicylic acid (DNS) method for determination of reducing sugar. An SIase from Erwinia sp. Ejp617 (ErSIase) was selected to improve its catalytic efficiency. After screening of ~ 8000 mutants from a random mutagenesis library, Q209 and R456 were identified as beneficial positions. Saturation mutagenesis of the two positions resulted in a double-site mutant ErSIase_Q209S-R456H that showed the highest catalytic efficiency, and its specific activity reached 684 U/mg that is 17.5-fold higher than that of the wild-type ErSIase. By employing the lyophilized Escherichia coli (E. coli) cells harboring ErSIase_Q209S-R456H, a high space–time yield (STY = 3.9 kg/(L·d)) was achieved toward 600 g/L sucrose. Furthermore, the in silico analysis suggested that the hydrogen bond network was improved and steric hindrance was reduced due to the beneficial substitutions.
Key points
• A sucrose isomerase mutant with high catalytic efficiency was obtained.
• The highest space–time yield was achieved toward high-concentration sucrose.
• The optimized H-bond network contributed to the enhanced catalytic efficiency.







Similar content being viewed by others
References
Aroonnual A, Nihira T, Seki T, Panbangred W (2007) Role of several key residues in the catalytic activity of sucrose isomerase from Klebsiella pneumoniae NK33-98-8. Enzyme Microb Technol 40:1221–1227
Arnold K, Bordoli L, Kopp J, Schwede T (2006) The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22:195–201
Cha J, Jung JH, Park SE, Cho MH, Seo DH, Ha SJ, Yoon JW, Lee OH, Kim YC, Park CS (2009) Molecular cloning and functional characterization of a sucrose isomerase (isomaltulose synthase) gene from Enterobacter sp. FMB-1. J Appl Microbiol 107:1119–1130
Cheetham PSJ (1984) The extraction and mechanism of a novel isomaltulose-synthesizing enzyme from Erwinia rhapontici. J Biochem 220:213–220
Cheng F, Chen Y, Qiu S, Zhai QY, Liu HT, Li SF, Weng CY, Wang YJ, Zheng YG (2021) Controlling stereopreferences of carbonyl reductases for enantioselective synthesis of atorvastatin precursor. ACS Catal 11:2572–2582
Cheng F, Tang XL, Kardashliev T (2018) Transcription Factor-Based Biosensors in High-Throughput Screening: Advances and Applications. Biotechnol J. 13(7):e1700648
Cheng F, Zhu L, Schwaneberg U (2015) Directed evolution 2.0: improving and deciphering enzyme properties. Chem Commun 51:9760–9772
Cheng F, Zhang JM, Jiang ZT, Wu XH, Xue YP, Zheng YG (2022) Development of an NAD(H)-driven biocatalytic system for asymmetric synthesis of chiral amino acids. Adv Synth Catal. https://doi.org/10.1002/adsc.202101441
Hamada S (2002) Role of sweeteners in the etiology and prevention of dental caries. Pure Appl Chem 74(7):1293–1300
Kawaguti HY, Celestino EM, Moraes ALL, Yim DK, Yamamoto LK, Sato HH (2010) Characterization of a glucosyltransferase from Erwinia sp. D12 and the conversion of sucrose into isomaltulose by immobilized cells. Biochem Eng J 48:211–217
Laksmi FA, Arai S, Tsurumaru H, Nakamura Y, Saksono B, Tokunaga M, Ishibashi M (2018) Improved substrate specificity for D-galactose of L-arabinose isomerase for industrial application. Biochim Biophys Acta Proteins Proteom 11:1084–1091
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) ClustalW and Clustal X version 2.0. Bioinformatics 23:2947–2948
Lee HC, Kim JH, Kim SY, Lee JK (2008) Isomaltose production by modification of the fructose-binding site on the basis of the predicted structure of sucrose isomerase from “Protaminobacter rubrum”. Appl Environ Microbiol 74:5183–5194
Li X, Zhao C, An Q, Zhang D (2003) Substrate induction of isomaltulose synthase in a newly isolated Klebsiella sp. LX3. J Appl Microbiol 95:521–527
Low NH, Sporns P (1988) Analysis and quantitation of minor di- and trisaccharides in honey, using capillary gas chromatography. J Food Sci 53(2):558–561
Markel U, Lanvers P, Sauer DF, Wittwer M, Dhoke GV, Davari MD, Schiffels J, Schwaneberg U (2021) A Photoclick-Based High-Throughput Screening for the Directed Evolution of Decarboxylase OleT. Chemistry 27(3):954–958
Mu W, Li W, Wang X, Zhang T, Jiang B (2014) Current studies on sucrose isomerase and biological isomaltulose production using sucrose isomerase. Appl Microbiol Biotechnol 98(15):6569–6582
Nagai Y, Sugitani T, Tsuyuki K (1994) Characterization of alpha-glucosyltransferase from Pseudomonas mesoacidophila MX-45. Biosci Biotechnol Biochem 58:1789–1793
Pilak P, Schiefner A, Seiboth J, Oehrlein J, Skerra A (2020) Engineering a Highly Active Sucrose Isomerase for Enhanced Product Specificity by Using a “Battleship” Strategy. ChemBioChem 21(15):2161–2169
Ren B, Li S, Xu H, Feng XH, Cai H, Ye Q (2011) Purification and characterization of a highly selective sucrose isomerase from Erwinia rhapontici NX-5. Bioprocess Biosyst Eng 34:629–637
Takazoe I (1985) New trends on sweeteners in Japan. Int Dent J 35:58–65
Véronèse T, Perlot P (1999) Mechanism of sucrose conversion by the sucrose isomerase of Serratia plymuthica ATCC 15928. Enzyme Microb Technol 24:263–269
Wu L, Birch RG (2005) Characterization of the highly efficient sucrose isomerase from Pantoea dispersa UQ68J and cloning of the sucrose isomerase gene. Appl Environ Microb 71(3):1581–1590
Wu L, Liu Y, Chi B, Xu Z, Feng X, Li S, Xu H (2015) An innovative method for immobilizing sucrose isomerase on epsilon-poly-L-lysine modified mesoporous TiO2. Food Chem 187:182–188
Wu L, Birch RG (2004) Characterization of Pantoea dispersa UQ68J: producer of a highly efficient sucrose isomerase for isomaltulose biosynthesis. J Appl Microbiol 97:93–103
Watzlawick H, Mattes R (2009) Gene cloning, protein characterization, and alteration of product selectivity for the trehalulose hydrolase and trehalulose synthase from “Pseudomonas mesoacidophila” MX-45. Appl Environ Microbiol 75:7026–7036
Zhang D, Li X, Zhang L-H (2002) Isomaltulose synthase from Klebsiella sp. strain LX3: gene cloning and characterization and engineering of thermostability. Appl Environ Microb 68(6):2676–2682
Zhang F, Cheng F, Jia DX, Gu YH, Liu ZQ, Zheng YG (2021) Characterization of a recombinant sucrose isomerase and its application to enzymatic production of isomaltulose. Biotechnol Lett 43(1):261–269
Zhang P, Wang ZP, Liu S, Wang YL, Zhang ZF, Liu XM, Du YM, Yuan XL (2019a) Overexpression of secreted sucrose isomerase in Yarrowia lipolytica and its application in isomaltulose production after immobilization. Int J Biol Macromol 121:97–103
Zhang YJ, Chen CS, Liu HT, Chen JL, Xia Y, Wu SJ (2019b) Purification, identification and characterization of an esterase with high enantioselectivity to (S)-ethyl indoline-2-carboxylate. Biotechnol Lett 41(10):1223–1232
Acknowledgements
This work was financially supported by the Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang, P. R. China (2018R01014) and the Zhejiang Provincial Qianjiang Talent Project.
Author information
Authors and Affiliations
Contributions
FZ and FC conceived and designed research. ZL and YZ supervised the project. FZ conducted experiments. FZ, DJ and QL analyzed data. FZ, FC and ZL wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, F., Cheng, F., Jia, DX. et al. Tuning the catalytic performances of a sucrose isomerase for production of isomaltulose with high concentration. Appl Microbiol Biotechnol 106, 2493–2501 (2022). https://doi.org/10.1007/s00253-022-11891-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-022-11891-5