Abstract
Aim
Mizoribine (MZR) is an immunosuppressant for the prevention of allograft rejection in Asian countries, but the great variability in pharmacokinetics (PK) limits its clinical use. This study was to explore genetic and clinical factors that affect the MZR PK process.
Methods
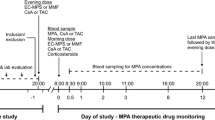
Blood samples and clinical data were collected from 60 Chinese renal transplant recipients. MZR plasma concentration was measured at pre-dose (0 h) and 0.5, 1, 2, 3, 4, 5, 6, 8, and 12 h post-dose by high performance liquid chromatography with an ultraviolet detector. PK parameters were calculated by non-compartmental analysis. High-throughput sequenced single nucleotide polymorphism was applied screening possible genetic factors.
Results
Extensive inter-individual MZR PK differences were reflected in the process of elimination (ke, CL/F, MRT and t1/2) and intestinal absorption (Cmax and Tmax), as well as in the dose-normalized exposure (AUC0–12h/D). From 146 SNPs within 39 genes screened, AUC0–12h/D was found higher in recipients with CREB1 rs11904814 TT than with G allele carriers (3.135 ± 0.928 versus 2.084 ± 0.379 μg h ml−1 mg−1, p = 0.007). Recipients with SLC28A3 rs10868138 TT had lower t1/2 as compared to C allele carriers (0.728 ± 0.189 versus 0.951 ± 0.196 h, p = 0.001). Serum creatinine (SCr) explained 35.5% of C0/D variability (p < 0.001). Pure effects of genotypes CREB1 and SLC28A3 were 13.7% (p = 0.004) and 17.5% (p = 0.001) for AUC0–12h/D and t1/2, respectively. When additionally taking SCr into models, CREB1 and SLC28A3 genotypes explained 20.0% (p = 0.038) and 46.5% (p < 0.001) of AUC0–12h/D and t1/2 variability, respectively.
Conclusion
CREB1 and SLC28A3 genotypes, as well as SCr, are identified as determinants in predicting inter-individual MZR PK differences in renal transplant recipients.




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Yokota S (2002) Mizoribine: mode of action and effects in clinical use. Pediatr Int 44(2):196–198. https://doi.org/10.1046/j.1328-8067.2002.01536.x
Yuan X, Chen C, Zheng Y, Wang C (2018) Conversion from mycophenolates to mizoribine is associated with lower BK virus load in kidney transplant recipients: a prospective study. Transplant Proc 50(10):3356–3360. https://doi.org/10.1016/j.transproceed.2018.01.059
Shi Y, Liu H, Chen XG, Shen ZY (2017) Comparison of mizoribine and mycophenolate mofetil with a tacrolimus-based immunosuppressive regimen in living-donor kidney transplantation recipients: a retrospective study in China. Transplant Proc 49(1):26–31. https://doi.org/10.1016/j.transproceed.2016.10.018
Ishida H, Takahara S, Amada N, Tomikawa S, Chikaraishi T, Takahashi K, Uchida K, Akiyama T, Tanabe K, Toma H (2016) A prospective randomized, comparative trial of high-dose mizoribine versus mycophenolate mofetil in combination with tacrolimus and basiliximab for living donor renal transplant: a multicenter trial. Exp Clin Transplant 14(5):518–525
Amemiya H, Suzuki S, Watanabe H, Hayashi R, Niiya S (1989) Synergistically enhanced immunosuppressive effect by combined use of cyclosporine and mizoribine. Transplant Proc 21(1 Pt 1):956–958
Thomson AW, Woo J, Yao GZ, Todo S, Starzl TE, Zeevi A (1993) Effects of combined administration of FK 506 and the purine biosynthesis inhibitors mizoribine or mycophenolic acid on lymphocyte DNA synthesis and T cell activation molecule expression in human mixed lymphocyte cultures. Transpl Immunol 1(2):146–150. https://doi.org/10.1016/0966-3274(93)90009-w
Tanabe K, Tokumoto T, Shimmura H, Toda F, Ishida H, Omoto K, Toma H (2002) Synergistic effect of high-dose mizoribine and low-dose tacrolimus on renal allograft survival in nonhuman primates. Transplant Proc 34(5):1428. https://doi.org/10.1016/s0041-1345(02)02914-7
Pouché L, Stojanova J, Marquet P, Picard N (2016) New challenges and promises in solid organ transplantation pharmacogenetics: the genetic variability of proteins involved in the pharmacodynamics of immunosuppressive drugs. Pharmacogenomics 17(3):277–296. https://doi.org/10.2217/pgs.15.169
Kawasaki Y (2009) Mizoribine: a new approach in the treatment of renal disease. Clin Dev Immunol 2009:681482–681410. https://doi.org/10.1155/2009/681482
Sonda K, Takahashi K, Tanabe K, Funchinoue S, Hayasaka Y, Kawaguchi H, Teraoka S, Toma H, Ota K (1996) Clinical pharmacokinetic study of mizoribine in renal transplantation patients. Transplant Proc 28(6):3643–3648
Liu D, Kobayashi T, Nagasaka T, Yokoyama I, Ma Y, Miwa Y, Kuzuya T, Morozumi K, Oikawa T, Shimano Y, Takeuchi O, Uchida K, Nakao A (2005) Potential value of high-dose mizoribine as rescue therapy for ongoing acute humoral rejection. Transplant Int 18(4):401–407. https://doi.org/10.1111/j.1432-2277.2004.00042.x
Chen P, Xu X, Liu L, Wu J, Li J, Fu Q, Chen J, Wang C (2019) Prediction of mizoribine pharmacokinetic parameters by serum creatinine in renal transplant recipients. Eur J Clin Pharmacol 75(3):363–369. https://doi.org/10.1007/s00228-018-2584-4
Liu L, Ren B, Zhang H, Li J, Fu Q, Jiang J, Deng S, Qiu J, Chen G, Fei J, Chen L, Wang C (2018) Population pharmacokinetic analysis of mizoribine in Chinese renal transplant recipients. Transplant Proc 50(8):2392–2397. https://doi.org/10.1016/j.transproceed.2018.03.030
Stypinski D, Obaidi M, Combs M, Weber M, Stewart AJ, Ishikawa H (2007) Safety, tolerability and pharmacokinetics of higher-dose mizoribine in healthy male volunteers. Br J Clin Pharmacol 63(4):459–468. https://doi.org/10.1111/j.1365-2125.2006.02779.x
Kaneda H, Shimizu M, Ohta K, Ushijima K, Gotoh Y, Satomura K, Nagai T, Fujieda M, Morooka M, Yamada T, Yamada M, Wada N, Takaai M, Hashimoto Y, Uemura O (2016) Population pharmacokinetics of mizoribine in pediatric patients with kidney disease. Clin Exp Nephrol 20(5):757–763. https://doi.org/10.1007/s10157-015-1209-9
Fukao M, Ishida K, Sakamoto T, Taguchi M, Matsukura H, Miyawaki T, Hashimoto Y (2011) Effect of genetic polymorphisms of SLC28A1, ABCG2, and ABCC4 on bioavailability of mizoribine in healthy Japanese males. Drug Metab Pharmacokinet 26(5):538–543. https://doi.org/10.2133/dmpk.dmpk-11-nt-040
Ishida K, Okamoto M, Ishibashi M, Hashimoto Y (2011) Population pharmacokinetics of mizoribine in adult recipients of renal transplantation. Clin Exp Nephrol 15(6):900–906. https://doi.org/10.1007/s10157-011-0487-0
Ihara H, Shinkuma D, Kubo M, Miyamoto I, Nojima M, Koike H, Yabumoto H, Ikoma F (1996) Influence of bioavailability on blood level of mizoribine in kidney transplant recipients. Transplant Proc 28(3):1321–1323
Mori N, Yokooji T, Kamio Y, Murakami T (2008) Characterization of intestinal absorption of mizoribine mediated by concentrative nucleoside transporters in rats. Eur J Pharmacol 586(1–3):52–58. https://doi.org/10.1016/j.ejphar.2008.02.043
Naito T, Tokashiki S, Mino Y, Otsuka A, Ozono S, Kagawa Y, Kawakami J (2010) Impact of concentrative nucleoside transporter 1 gene polymorphism on oral bioavailability of mizoribine in stable kidney transplant recipients. Basic Clin Pharmacol Toxicol 106(4):310–316. https://doi.org/10.1111/j.1742-7843.2009.00489.x
Ishida K, Takaai M, Yotsutani A, Taguchi M, Hashimoto Y (2009) Membrane transport mechanisms of mizoribine in the rat intestine and human epithelial LS180 cells. Biol Pharm Bull 32(4):741–745. https://doi.org/10.1248/bpb.32.741
Ren B, Fu XH, Zhang ZH, Huang L, Wang CX, Chen X (2013) Determination of mizoribine in human plasma using high-performance liquid chromatography: application to a pharmacokinetic study in Chinese renal transplant recipients. Drug Res 63(7):376–381. https://doi.org/10.1055/s-0033-1341499
Oshiro Y, Nakagawa K, Hoshinaga K, Aikawa A, Shishido S, Yoshida K, Asano T, Murai M, Hasegawa A (2013) A Japanese multicenter study of high-dose mizoribine combined with cyclosporine, basiliximab, and corticosteroid in renal transplantation (the fourth report). Transplant Proc 45(4):1476–1480. https://doi.org/10.1016/j.transproceed.2013.03.016
Nishioka T, Yoshimura N, Ushigome H, Watarai Y, Nishimura K, Akioka K, Nakamura N, Kawakita M, Yuzawa K, Nakatani T (2018) High-dose mizoribine combined with calcineurin inhibitor (cyclosporine or tacrolimus), basiliximab and corticosteroids for renal transplantation: a Japanese multicenter study. Int J Urology 25(2):141–145. https://doi.org/10.1111/iju.13476
Kitazawa S, Kondo T, Mori K, Yokoyama N, Matsuo M, Kitazawa R (2012) A p.D116G mutation in CREB1 leads to novel multiple malformation syndrome resembling CrebA knockout mouse. Hum Mutat 33(4):651–654. https://doi.org/10.1002/humu.22027
Xu Y, Song R, Long W, Guo H, Shi W, Yuan S, Xu G, Zhang T (2018) CREB1 functional polymorphisms modulating promoter transcriptional activity are associated with type 2 diabetes mellitus risk in Chinese population. Gene 665:133–140. https://doi.org/10.1016/j.gene.2018.05.002
Y-h D, Ma J, Wang L, Yang Y, Qiao Z, Fang D, Qiu X, Yang X, Zhu X, He J, Pan H, Ban B, Zhao Y, Sui H (2017) GNB3 and CREB1 gene polymorphisms combined with negative life events increase susceptibility to major depression in a Chinese Han population. PLoS One 12(2):e0170994. https://doi.org/10.1371/journal.pone.0170994
Hettema JM, An S-S, van den Oord EJCG, Neale MC, Kendler KS, Chen X (2009) Association study of CREB1 with major depressive disorder and related phenotypes. Am J Med Genet B Neuropsychiatr Genet 150B(8):1128–1132. https://doi.org/10.1002/ajmg.b.30935
Shan Q, Zheng G, Zhu A, Cao L, Lu J, Wu D, Zhang Z, Fan S, Sun C, Hu B, Zheng Y (2016) Epigenetic modification of miR-10a regulates renal damage by targeting CREB1 in type 2 diabetes mellitus. Toxicol Appl Pharmacol 306:134–143. https://doi.org/10.1016/j.taap.2016.06.010
Godoy V, Banales JM, Medina JF, Pastor-Anglada M (2014) Functional crosstalk between the adenosine transporter CNT3 and purinergic receptors in the biliary epithelia. J Hepatol 61(6):1337–1343. https://doi.org/10.1016/j.jhep.2014.06.036
Choi DS, Handa M, Young H, Gordon AS, Diamond I, Messing RO (2000) Genomic organization and expression of the mouse equilibrative, nitrobenzylthioinosine-sensitive nucleoside transporter 1 (ENT1) gene. Biochem Biophys Res Commun 277(1):200–208. https://doi.org/10.1006/bbrc.2000.3665
Nam HW, Hinton DJ, Kang NY, Kim T, Lee MR, Oliveros A, Adams C, Ruby CL, Choi DS (2013) Adenosine transporter ENT1 regulates the acquisition of goal-directed behavior and ethanol drinking through A2A receptor in the dorsomedial striatum. J Neurosci 33(10):4329–4338. https://doi.org/10.1523/jneurosci.3094-12.2013
Young JD, Yao SY, Baldwin JM, Cass CE, Baldwin SA (2013) The human concentrative and equilibrative nucleoside transporter families, SLC28 and SLC29. Mol Asp Med 34(2–3):529–547. https://doi.org/10.1016/j.mam.2012.05.007
Podgorska M, Kocbuch K, Pawelczyk T (2005) Recent advances in studies on biochemical and structural properties of equilibrative and concentrative nucleoside transporters. Acta Biochim Pol 52(4):749–758
Fernández-Calotti P, Casulleras O, Antolin M, Guarner F, Pastor-Anglada M (2016) Galectin-4 interacts with the drug transporter human concentrative nucleoside transporter 3 to regulate its function. FASEB J 30(2):544–554. https://doi.org/10.1096/fj.15-272773
Casado FJ, Lostao MP, Aymerich I, Larráyoz IM, Duflot S, Rodríguez-Mulero S, Pastor-Anglada M (2002) Nucleoside transporters in absorptive epithelia. J Physiol Biochem 58(4):207–216. https://doi.org/10.1007/bf03179858
Pastor-Anglada M, Cano-Soldado P, Errasti-Murugarren E, Casado FJ (2008) SLC28 genes and concentrative nucleoside transporter (CNT) proteins. Xenobiotica 38(7–8):972–994. https://doi.org/10.1080/00498250802069096
Feng Y, Wang C, Liu Q, Meng Q, Huo X, Liu Z, Sun P, Yang X, Sun H, Qin J, Liu K (2016) Bezafibrate-mizoribine interaction: involvement of organic anion transporters OAT1 and OAT3 in rats. Eur J Pharm Sci 81:119–128. https://doi.org/10.1016/j.ejps.2015.10.008
Utsunomiya Y, Hara Y, Ito H, Okonogi H, Miyazaki Y, Hashimoto Y, Hosoya T (2010) Effects of probenecid on the pharmacokinetics of mizoribine and co-administration of the two drugs in patients with nephrotic syndrome. Int J Clin Pharmacol Ther 48(11):751–755. https://doi.org/10.5414/cpp48751
Burckhardt G (2012) Drug transport by organic anion transporters (OATs). Pharmacol Ther 136(1):106–130. https://doi.org/10.1016/j.pharmthera.2012.07.010
Funding
This study was financially supported by Guangdong Basic and Applied Basic Research Foundation (No. 2020A1515010138), Young Teacher Foundation of Sun Yat-sen University (19ykpy79, 19ykpy04), and National Key R&D Program of China (2017YFC0909900).
Author information
Authors and Affiliations
Contributions
Conception and design: Pan Chen, Xiao Chen.
Acquisition of data: Qian Fu, Changxi Wang.
Analysis and interpretation of the data: Rui Dai, Jingjie Li, Jingjing Wu, Guoping Zhong.
Drafting of the article: Rui Dai, Pan Chen, Jingjie Li.
Critical review: All authors.
Corresponding authors
Ethics declarations
This study was approved by the ethics committee of the First Affiliated Hospital of Sun Yat-sen University (approved no: 2015118), and informed consent was obtained from each enrolled patient.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 118 kb)
Rights and permissions
About this article
Cite this article
Dai, R., Li, J., Wu, J. et al. Genetic and clinical determinants of mizoribine pharmacokinetics in renal transplant recipients. Eur J Clin Pharmacol 77, 45–53 (2021). https://doi.org/10.1007/s00228-020-02936-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-020-02936-7