Abstract
Purpose
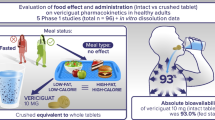
Fostamatinib is an orally dosed phosphate prodrug that is cleaved by intestinal alkaline phosphatase to the active metabolite R406. Clinical studies were performed to assess the effect of food and ranitidine on exposure, to support in vitro-in vivo relationships (IVIVR) understanding and formulation transitions and to investigate absolute oral bioavailability.
Methods
A series of in vitro dissolution and clinical pharmacokinetic studies were performed to support the design and introduction of a new formulation, understand the impact of changes in in vitro dissolution on in vivo performance for two fostamatinib formulations, to characterize the effects of food and ranitidine on exposure, and determine the absolute oral bioavailability.
Results
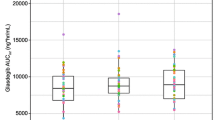
The in vivo performance of fostamatinib was generally insensitive to changes in in vitro dissolution performance, although marked slowing of the dissolution rate did impact exposures. Food and ranitidine had minor effects on R406 exposure that were not considered clinically relevant. The absolute oral bioavailability of fostamatinib was 54.6 %.
Conclusions
The absolute oral bioavailability of fostamatinib was ~55 %. Food and ranitidine had minor effects on R406 exposure. An in vitro dissolution versus clinical performance relationship was determined that supported formulation transitions.




Similar content being viewed by others
References
Sweeny DJ, Li W, Clough J, Bhamidipati S, Singh R, Park G, Baluom M, Grossbard E, Lau DT (2010) Metabolism of fostamatinib, the oral methylene phosphate prodrug of the spleen tyrosine kinase inhibitor R406 in humans: contribution of hepatic and gut bacterial processes to the overall biotransformation. Drug Metab Dispos 38:1166–1176
Dawes P, Dimic A, Genovese MC et al (2013) OSKIRA-2: a phase III, multicenter, randomized, double-blind, placebo-controlled parallel-group study of 2 dosing regimens of fostamatinib in rheumatoid arthritis patients with an inadequate response to disease-modifying antirheumatic drugs. Arthritis Rheum 65:S198
Genovese MC, van der Heijde D, Keystone E et al (2013) OSKIRA-3: a phase III, multicenter, randomized, double-blind, placebo-controlled parallel-group study of 2 dosing regimens of fostamatinib in rheumatoid arthritis patients with an inadequate response to a tumor necrosis factor-alpha antagonist. Arthritis Rheum 65:S199
Taylor PC, Genovese MC, Greenwood M et al (2013) OSKIRA-4: a phase IIB randomised, placebo-controlled study of the efficacy and safety of fostamatinib monotherapy. Ann Rheum Dis 72(Suppl 3):66
Baluom M, Grossbard EB, Mant T, Lau DT (2013) Pharmacokinetics of fostamatinib, a spleen tyrosine kinase (SYK) inhibitor, in healthy human subjects following single and multiple oral dosing in three phase I studies. Br J Clin Pharmacol 76:78–88
Wire MB, Shelton MJ, Studenberg S (2006. Fosamprenavir clinical pharmacokinetics and drug interactions of the amprenavir prodrug. Clin Pharmacokinet 45(2):137–168
Acknowledgments
We acknowledge Paul Severin at Covance for performing the R406 bioanalytical work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were approved by the independent Institutional Review Boards and in accordance with applicable regulatory requirements, Good Clinical Practice guidelines, and ethical principles that have their origins in the Declaration of Helsinki. Informed consent was obtained from all subjects prior to initiation of the studies.
Conflict of interest
Michael Gillen is an employee of AstraZeneca and hold stocks/shares in the company. Talia Flanagan is an employee of AstraZeneca. David Mathews has no conflicts of interest to declare. Eleanor Lisbon is an employee of Quintiles and holds stock/shares in the company. Martin Kruusmägi and Paul Martin are ex-employees of AstraZeneca and holds stocks/shares in the company.
Authors’ contributions
TF and PM had full access to all of the data in the studies and take responsibility for the integrity of the data and the accuracy of the data analysis. Additionally, TF, PM, and MG were involved with the study concept and design. PM and MG participated in the acquisition of data. Analysis and interpretation of data was carried out by PM and MG. All authors participated in article preparation and provided final approval of the manuscript.
Funding
These studies were sponsored by AstraZeneca.
Rights and permissions
About this article
Cite this article
Flanagan, T., Martin, P., Gillen, M. et al. Effects of ranitidine (antacid), food, and formulation on the pharmacokinetics of fostamatinib: results from five phase I clinical studies. Eur J Clin Pharmacol 73, 185–195 (2017). https://doi.org/10.1007/s00228-016-2156-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-016-2156-4