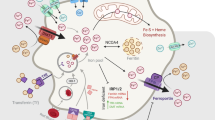
Abstract
Innate immune systems alter the concentrations of trace elements in host niches in response to invading pathogens during infection. This work reports the interplay between d-block metal ions and their associated biomolecules using hyphenated elemental techniques to spatially quantify both elemental distributions and the abundance of specific transport proteins. Here, lung tissues were collected for analyses from naïve and Streptococcus pneumoniae-infected mice fed on a zinc-restricted or zinc-supplemented diet. Spatiotemporal distributions of manganese (55Mn), iron (56Fe), copper (63Cu), and zinc (66Zn) were determined by quantitative laser ablation–inductively coupled plasma–mass spectrometry. The murine transport proteins ZIP8 and ZIP14, which are associated with zinc transport, were also imaged by incorporation of immunohistochemistry techniques into the analytical workflow. Collectively, this work demonstrates the potential of a single instrumental platform suitable for multiplex analyses of tissues and labelled antibodies to investigate complex elemental interactions at the host-pathogen interface. Further, these methods have the potential for broad application to investigations of biological pathways where concomitant measurement of elements and biomolecules is crucial to understand the basis of disease and aid in development of new therapeutic approaches.






Similar content being viewed by others
References
Palmer LD, Skaar EP. Transition metals and virulence in bacteria. Annu Rev Genet. 2016;50:67–91. https://doi.org/10.1146/annurev-genet-120215-035146.
Johnson MDL, Kehl-Fie TE, Klein R, Kelly J, Burnham C, Mann B, Rosch JW. Role of copper efflux in pneumococcal pathogenesis and resistance to macrophage-mediated immune clearance. Infect Immun. 2015;83:1684–94. https://doi.org/10.1128/IAI.03015-14.
Porcheron G, Garénaux A, Proulx J, Sabri M, Dozois CM. Iron, copper, zinc, and manganese transport and regulation in pathogenic Enterobacteria: correlations between strains, site of infection and the relative importance of the different metal transport systems for virulence. Front Cell Infect Microbiol. 2013;3:1–24. https://doi.org/10.3389/fcimb.2013.00090.
WHO. Treatment and prevention of pneumonia. World Health Assambly. 2010;63 (ed.):1–4.
Honsa ES, Johnson MDL, Rosch JW. The roles of transition metals in the physiology and pathogenesis of Streptococcus pneumoniae. Front Cell Infect Microbiol. 2013;3:1–15. https://doi.org/10.3389/fcimb.2013.00092.
Eijkelkamp BA, Morey JR, Neville SL, Tan A, Pederick VG, Cole N, Singh PP, Ong C-LY, Gonzalez de Vega R, Clases D, Cunningham BA, Hughes CE, Comerford I, Brazel EB, Whittall JJ, Plumptre CD, McColl SR, Paton JC, McEwan AG, Doble PA, McDevitt CA. Dietary zinc and the control of Streptococcus pneumoniae infection. PLoS Pathog. 2019;15:e1007957. https://doi.org/10.1371/journal.ppat.1007957.
Neville SL, Sjöhamn J, Watts JA, MacDermott-Opeskin H, Fairweather SJ, Ganio K, Carey Hulyer A, McGrath AP, Hayes AJ, Malcolm TR, Davies MR, Nomura N, Iwata S, O ML, Maher MJ, McDevitt CA. The structural basis of bacterial manganese import.2021
McDevitt CA, Ogunniyi AD, Valkov E, Lawrence MC, Kobe B, McEwan AG, Paton JC. A molecular mechanism for bacterial susceptibility to Zinc. PLoS Pathog. 2011;7 https://doi.org/10.1371/journal.ppat.1002357
Eijkelkamp BA, Morey JR, Ween MP, Ong CLY, McEwan AG, Paton JC, McDevitt CA. Extracellular zinc competitively inhibits manganese uptake and compromises oxidative stress management in Streptococcus pneumoniae. PLoS One. 2014;9 https://doi.org/10.1371/journal.pone.0089427
Doble PA, Miklos GLG. Distributions of manganese in diverse human cancers provide insights into tumour radioresistance. Metallomics. 2018;10:1191–210. https://doi.org/10.1039/c8mt00110c.
Morona JK, Morona R, Miller DC, Paton JC. Streptococcus pneumoniae capsule biosynthesis protein CpsB is a novel manganese-dependent phosphotyrosine-protein phosphatase. J Bacteriol. 2002;184:577–83. https://doi.org/10.1128/JB.184.2.577-583.2002.
Hoyer J, Bartel J, Gómez-Mejia A, Rohde M, Hirschfeld C, Heß N, Sura T, Maaß S, Hammerschmidt S, Becher D. Proteomic response of Streptococcus pneumoniae to iron limitation. Int J Med Microbiolog. 2018;308:713–21. https://doi.org/10.1016/j.ijmm.2018.02.001.
Jenkitkasemwong S, Wang CY, MacKenzie B, Knutson MD. Physiologic implications of metal-ion transport by ZIP14 and ZIP8. BioMetals. 2012;25:643–55. https://doi.org/10.1007/s10534-012-9526-x.
Bafaro E, Liu Y, Xu Y, Dempski RE. The emerging role of zinc transporters in cellular homeostasis and cancer. Signal Transduct Target Ther. 2017;2:1–12. https://doi.org/10.1038/sigtrans.2017.29.
Liu MJ, Bao S, Gálvez-Peralta M, Pyle CJ, Rudawsky AC, Pavlovicz RE, Killilea DW, Li C, Nebert DW, Wewers MD, Knoell DL. ZIP8 regulates host defense through zinc-mediated inhibition of NF-κB. Cell Rep. 2013;3:386–400. https://doi.org/10.1016/j.celrep.2013.01.009.
Besecker B, Bao S, Bohacova B, Papp A, Sadee W, Knoell DL. The human zinc transporter SLC39A8 (Zip8) is critical in zinc-mediated cytoprotection in lung epithelia. Am J Physiol Lung Cell Mol Physiol. 2008;294:1127–36. https://doi.org/10.1152/ajplung.00057.2008.
Wessels I, Cousins RJ. Zinc dyshomeostasis during polymicrobial sepsis in mice involves zinc transporter Zip14 and can be overcome by zinc supplementation. Am J Physiol Gastrointest Liver Physiol. 2015;309:768–78. https://doi.org/10.1152/ajpgi.00179.2015.
Sapkota M, Knoell DL (2018) Essential role of zinc and zinc transporters in myeloid cell function and host defense against infection. J Immunol Res 2018:. https://doi.org/10.1155/2018/4315140
Hara T, Takeda T, aki, Takagishi T, Fukue K, Kambe T, Fukada T,. Physiological roles of zinc transporters: molecular and genetic importance in zinc homeostasis. J Physiolog Sci. 2017;67:283–301. https://doi.org/10.1007/s12576-017-0521-4.
Zang ZS, Xu YM, Lau ATY. Molecular and pathophysiological aspects of metal ion uptake by the zinc transporter ZIP8 (SLC39A8). Toxicol Res (Camb). 2016;5:987–1002. https://doi.org/10.1039/c5tx00424a.
Doble PA, Gonzalez de Vega R, Bishop DP, Hare DJ, Clases D. Laser ablation-inductively coupled plasma-mass spectrometry imaging in biology. Chem Rev. 2021. https://doi.org/10.1021/acs.chemrev.0c01219.
Bishop DP, Hare DJ, Clases D, Doble PA. Applications of liquid chromatography-inductively coupled plasma-mass spectrometry in the biosciences: a tutorial review and recent developments. TrAC - Trends Analy Chem. 2018;104:11–21.
Clases D, Fingerhut S, Jeibmann A, Sperling M, Doble P, Karst U. LA-ICP-MS/MS improves limits of detection in elemental bioimaging of gadolinium deposition originating from MRI contrast agents in skin and brain tissues. J Trace Elements Med Biolog. 2019;51:212–8. https://doi.org/10.1016/j.jtemb.2018.10.021.
Clases D, Gonzalez De Vega R, Adlard PA, Doble PA. On-line reverse isotope dilution analysis for spatial quantification of elemental labels used in immunohistochemical assisted imaging mass spectrometry via LA-ICP-MS. J Anal At Spectrom. 2019;34:407–12. https://doi.org/10.1039/c8ja00324f.
Hare DJ, Lear J, Bishop D, Beavis A, Doble P, a. Protocol for production of matrix-matched brain tissue standards for imaging by laser ablation-inductively coupled plasma-mass spectrometry. Analytical Meth. 2013;5:1915. https://doi.org/10.1039/c3ay26248k.
Šala M, Šelih VS, van Elteren JT. Gelatin gels as multi-element calibration standards in LA-ICP-MS bioimaging: fabrication of homogeneous standards and microhomogeneity testing. Analyst. 2017;142:3356–9. https://doi.org/10.1039/C7AN01361B.
Waentig L, Jakubowski N, Hardt S, Scheler C, Roos PH, Linscheid MW. Comparison of different chelates for lanthanide labeling of antibodies and application in a Western blot immunoassay combined with detection by laser ablation (LA-)ICP-MS. J Anal At Spectrom. 2012;27:1311–20. https://doi.org/10.1039/c2ja30068k.
Liu R, Wu P, Yang L, Hou X, Lv Y. Inductively coupled plasma mass spectrometry-based immunoassay: a review. Mass Spectrom Rev. 2014;33:373–93. https://doi.org/10.1002/mas.21391.
Seuma J, Bunch J, Cox A, McLeod C, Bell J, Murray C. Combination of immunohistochemistry and laser ablation ICP mass spectrometry for imaging of cancer biomarkers. Proteomics. 2008;8:3775–84. https://doi.org/10.1002/pmic.200800167.
Bishop DP, Cole N, Zhang T, Doble PA, Hare DJ. A guide to integrating immunohistochemistry and chemical imaging. Chem Soc Rev. 2018;47:3770–87. https://doi.org/10.1039/c7cs00610a.
Clases D, Gonzalez de Vega R, Funke S, Lockwood TE, Westerhausen M, Taudte RV, Adlard PA, Doble P. Matching sensitivity to abundance: high resolution immuno-mass spectrometry imaging of lanthanide labels and endogenous elements in the murine brain. J Anal At Spectrom. 2020;35:728–35. https://doi.org/10.1039/C9JA00405J.
Hare DJ, Lei P, Ayton S, Roberts BR, Grimm R, George JL, Bishop DP, Beavis AD, Donovan SJ, McColl G, Volitakis I, Masters CL, Adlard Pa, Cherny Ra, Bush AI, Finkelstein DI, Doble Pa. An iron–dopamine index predicts risk of parkinsonian neurodegeneration in the substantia nigra pars compacta. Chem Sci. 2014;5:2160. https://doi.org/10.1039/c3sc53461h.
Giesen C, Wang HAO, Schapiro D, Zivanovic N, Jacobs A, Hattendorf B, Schüffler PJ, Grolimund D, Buhmann JM, Brandt S, Varga Z, Wild PJ, Günther D, Bodenmiller B. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat Meth. 2014;11:417–22. https://doi.org/10.1038/nmeth.2869.
Ramos-Vara JA. Technical aspects of immunohistochemistry. Vet Pathol. 2005;42:405–26. https://doi.org/10.1354/vp.42-4-405.
Kim SW, Roh J, Park CS. Immunohistochemistry for pathologists: protocols, pitfalls, and tips. J Pathol Transl Med. 2016;50:411–8.
Brey EM, Lalani Z, Johnston C, Wong M, McIntire LV, Duke PJ, Patrick CW. Automated selection of DAB-labeled tissue for immunohistochemical quantification. J Histochem Cytochem. 2003;51:575–84. https://doi.org/10.1177/002215540305100503.
Westerhausen MT, Lockwood TE, Gonzalez De Vega R, Röhnelt A, Bishop DP, Cole N, Doble PA, Clases D. Low background mould-prepared gelatine standards for reproducible quantification in elemental bio-imaging. Analyst. 2019;144:6881–8. https://doi.org/10.1039/c9an01580a.
Lockwood TE, Westerhausen MT, Doble PA. Pew2: open-source imaging software for laser ablation-inductively coupled plasma-mass spectrometry. Anal Chem. 2021;93:10418–23. https://doi.org/10.1021/acs.analchem.1c02138.
Brazel EB, Tan A, Neville SL, Iverson AR, Udagedara SR, Cunningham BA, Sikanyika M, De Oliveira DMP, Keller B, Bohlmann L, El-Deeb IM, Ganio K, Eijkelkamp BA, McEwan AG, von Itzstein M, Maher MJ, Walker MJ, Rosch JW, McDevitt CA. Dysregulation of Streptococcus pneumoniae zinc homeostasis breaks ampicillin resistance in a pneumonia infection model. Cell Rep. 2022;38:1010202. https://doi.org/10.1016/j.celrep.2021.110202.
Clases D, Gonzalez de Vega R, Bishop D, Doble P. SEC-ICP-MS and on-line isotope dilution analysis for characterisation and quantification of immunochemical assays. Anal Bioanal Chem. 2019;411:3553–60. https://doi.org/10.1007/s00216-019-01836-9.
Rodríguez-González P, Marchante-Gayón JM, García Alonso JI, Sanz-Medel A. Isotope dilution analysis for elemental speciation: a tutorial review. Spectrochimica Acta - Part B. 2005;60:151–207. https://doi.org/10.1016/j.sab.2005.01.005.
Otsu N. A threshold selection method from gray-level histograms. IEEE Trans Syst Man Cybern. 1979;9:62–6. https://doi.org/10.1109/TSMC.1979.4310076.
Bishop DP, Westerhausen MT, Barthelemy F, Lockwood T, Cole N, Gibbs EM, Crosbie RH, Nelson SF, Miceli MC, Doble PA, Wanagat J. Quantitative immuno-mass spectrometry imaging of skeletal muscle dystrophin. Sci Rep. 2021;11:1128. https://doi.org/10.1038/s41598-020-80495-8.
Neville SL, Cunningham BA, Maunders EA, Tan A, Watts JA, Ganio K, Eijkelkamp BA, Pederick VG, Gonzalez de Vega R, Clases D, Doble PA, McDevitt CA. Host-mediated copper stress is not protective against Streptococcus pneumoniae D39 infection. Microbiol Spectr. 2022:10 https://doi.org/10.1128/spectrum.02495-22
Cassat JE, Skaar EP. Iron in infection and immunity. Cell Host Microbe. 2013;13:509–19. https://doi.org/10.1016/j.chom.2013.04.010.
Muckenthaler MU, Rivella S, Hentze MW, Galy B. A red carpet for iron metabolism. Cell. 2017;168:344–61.
Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–6. https://doi.org/10.1172/JCI200420945.
Rhew K, Choi J, Kim K, Choi KH, Lee SH, Park HW. Increased risk of anemia in patients with asthma. Clin Epidemiol. 2023;15:31–8. https://doi.org/10.2147/CLEP.S394717.
Bowers K, Srai SKS. The trafficking of metal ion transporters of the Zrt- and Irt-like protein family. Traffic. 2018;19:813–22. https://doi.org/10.1111/tra.12602.
Wang CY, Jenkitkasemwong S, Duarte S, Sparkman BK, Shawki A, Mackenzie B, Knutson MD. ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading. J Biolog Chem. 2012;287:34032–43. https://doi.org/10.1074/jbc.M112.367284.
Scheiber IF, Alarcon NO, Zhao N. Manganese uptake by A549 cells is mediated by both ZIP8 and ZIP14. Nutrients. 2019:11 https://doi.org/10.3390/nu11071473
Pyle CJ, Azad AK, Papp AC, Sadee W, Knoell DL, Schlesinger LS. Elemental ingredients in the macrophage cocktail: role of zip8 in host response to Mycobacterium tuberculosis. Int J Mol Sci. 2017:18 https://doi.org/10.3390/ijms18112375
Zhang V, Ganz T, Nemeth E, Kim A. The role of the iron transporter Zip8 in pulmonary host defense. Am J Respir Crit Care Med. 2020;201:A7437. https://doi.org/10.1164/ajrccm-conference.2020.201.1_meetingabstracts.a7437.
Funding
This work was supported by the Australian Research Council (ARC) Discovery Project grants DP190102361 to PAD and DP220100713 to CAM. SLN is supported by a Passe & Williams Foundation Junior Fellowship. Philanthropic support of the AMI by the Miklos family is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published in the topical collection Elemental Mass Spectrometry for Bioanalysis with guest editors Jörg Bettmer, Mario Corte-Rodríguez, and Márcia Foster Mesko.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gonzalez de Vega, R., Clases, D., Cunningham, B.A. et al. Spatial distribution of trace metals and associated transport proteins during bacterial infection. Anal Bioanal Chem 416, 2783–2796 (2024). https://doi.org/10.1007/s00216-023-05068-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-023-05068-w