Abstract
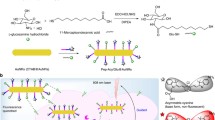
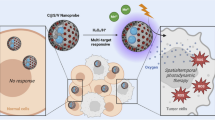
Despite significant advancements in cancer research, real-time monitoring and effective treatment of cancer through non-invasive techniques remain a challenge. Herein, a novel polydopamine (PDA) nucleic acid nanoprobe has been developed for imaging signal amplification of intracellular mRNA and precise photothermal therapy guidance in cancer cells. The PDA nucleic acid nanoprobe (PDA@DNA) is constructed by assembling an aptamer hairpin (H1) labeled with the Cy5 fluorophore and another nucleic acid recognition hairpin (H2) onto PDA nanoparticles (PDA NPs), which have exceptionally high fluorescence quenching ability and excellent photothermal conversion properties. The nanoprobe could facilitate cellular uptake of DNA molecules and their protection from nuclease degradation. Upon recognition and binding to the intracellular mRNA target, a catalytic hairpin assembly (CHA) reaction occurs. The stem of H1 unfolds upon binding, allowing the exposed H1 to hybridize with H2, forming a flat and sturdy DNA double-stranded structure that detaches from the surface of PDA NPs. At the same time, the target mRNA is displaced and engages in a new cyclic reaction, resulting in the recovery and significant amplification of Cy5 fluorescence. Using thymidine kinase1 (TK1) mRNA as a model mRNA, this nanoprobe enables the analysis of TK1 mRNA with a detection limit of 9.34 pM, which is at least two orders of magnitude lower than that of a non-amplifying imaging nucleic acid probe. Moreover, with its outstanding performance for in vitro detection, this nanoprobe excels in precisely imaging tumor cells. Through live-cell TK1 mRNA imaging, it can accurately distinguish between tumor cells and normal cells. Furthermore, when exposed to 808-nm laser irradiation, the nanoprobe fully harnesses exceptional photothermal conversion properties of PDA NPs. This results in a localized temperature increase within tumor cells, which ultimately triggers apoptosis in these tumor cells. The integration of PDA@DNA presents innovative prospects for tumor diagnosis and image-guided tumor therapy, offering the potential for high-precision diagnosis and treatment of tumors.
Graphical Abstract






Similar content being viewed by others
References
Haider M, Zaki KZ, Hamshary MRE, Hussain Z, Orive G, Ibrahim HO. Polymeric nanocarriers: a promising tool for early diagnosis and efficient treatment of colorectal cancer. J Adv Res. 2022;39:237–55.
McWilliams A, Lam B, Sutedja T. Early proximal lung cancer diagnosis and treatment. Eur Respir J. 2009;33:656–65.
Yang J, Xu R, Wang C, Qiu J, Ren B, You L. Early screening and diagnosis strategies of pancreatic cancer: a comprehensive review. Cancer Commun. 2021;41:1257–74.
Thakur SK, Singh DP, Choudhary J. Lung cancer identification: a review on detection and classification. Cancer Metast Rev. 2020;39:989–98.
Njoku K, Chiasserini D, Whetton AD, Crosbie EJ. Proteomic biomarkers for the detection of endometrial cancer. Cancers. 2019;11:1572.
McGee JP, Su P, Durbin KR, Hollas MA, Bateman NW, Maxwell GL, Conrads TP, Fellers RT, Melani RD, Camarillo JM, Kafader JO, Kelleher NL. Automated imaging and identification of proteoforms directly from ovarian cancer tissue. Nat Commun. 2023;14:6478.
Waks AG, Winer EP. Breast cancer treatment: a review. Jama. 2019;321:288–300.
Kuroki L, Guntupalli SR. Treatment of epithelial ovarian cancer. Bmj. 2020;371: m3773.
Cullen JK, Simmons JL, Parsons PG, Boyle GM. Topical treatments for skin cancer. Adv Drug Deliver Rev. 2020;153:54–64.
Ishaqat A, Herrmann A. Polymers strive for accuracy: from sequence-defined polymers to mRNA vaccines against COVID-19 and polymers in nucleic acid therapeutics. J Am Chem Soc. 2021;143:20529–45.
Binzel DW, Li X, Burns N, Khan E, Lee WJ, Chen LC, Ellipilli S, Miles W, Ho YS, Guo P. Thermostability, tunability, and tenacity of RNA as rubbery anionic polymeric materials in nanotechnology and nanomedicine-specific cancer targeting with undetectable toxicity. Chem Rev. 2021;121:7398–467.
Bornewasser L, Domnick C, Kath-Schorr S. Stronger together for in-cell translation: natural and unnatural base modified mRNA. Chem Sci. 2022;13:4753–61.
Fleming SR, Himes PM, Ghodge SV, Goto Y, Suga H, Bowers AA. Exploring the post-translational enzymology of PaaA by mRNA display. J Am Chem Soc. 2020;142:5024–8.
Liu C, Deng J, Yi J, Zhang R, Chen L, Fu X, Liao S, Wenjun Yi G, Yang H. A novel binding-induced DNAzyme motor triggered by survivin mRNA. Anal Bioanal Chem. 2022;414:6167–75.
Lin M, Yi X, Huang F, Ma X, Zuo X, Xia F. Photoactivated nanoflares for mRNA detection in single living cells. Anal Chem. 2019;91:2021–7.
Li X, Wu Y, Zhang X, Liu J, Zhang Y, Yuan L, Liu M. Thermodynamic and cellular studies of doxorubicin/daunorubicin loaded by a DNA tetrahedron for diagnostic imaging, chemotherapy, and gene therapy. Int J Biol Macromol. 2023;251: 126245.
Krissanaprasit A, Key CM, Pontula S, LaBean TH. Self-assembling nucleic acid nanostructures functionalized with aptamers. Chem Rev. 2021;121:13797–868.
Yin X, Chen B, He M, Hu B. A multifunctional platform for the capture, release, and enumeration of circulating tumor cells based on aptamer binding, nicking endonuclease-assisted amplification, and inductively coupled plasma mass spectrometry detection. Anal Chem. 2020;92:10308–15.
Jou AFJ, Chou YT, Willner I, Ho JA. Imaging of cancer cells and dictated cytotoxicity using aptamer-guided hybridization chain reaction (HCR)-generated g-quadruplex chains. Angew Chem Int Ed. 2021;60:21673–8.
Liu XQ, Wang T, Wu YW, Tan YF, Jiang T, Li K, Lou BB, Chen LW, Liu YF, Liu ZB. Aptamer based probes for living cell intracellular molecules detection. Biosens Bioelectron. 2022;208: 114231.
He M, He M, Nie C, Yi J, Zhang J, Chen T, Chu X. mRNA-activated multifunctional DNAzyme nanotweezer for intracellular mRNA sensing and gene therapy. ACS Appl Mater Inter. 2021;13:8015–25.
Gao P, Shen X, Liu X, Chen Y, Pan W, Li N, Tang B. Nucleic acid-gated covalent organic frameworks for cancer-specific imaging and drug release. Anal Chem. 2021;93:11751–7.
Egloff S, Melnychuk N, Cruz Da Silva E, Reisch A, Martin S, Klymchenko A S. Amplified fluorescence in situ hybridization by small and bright dye-loaded polymeric nanoparticles. ACS Nano. 2021;16:1381–1394.
Du H, Li X, Xu S, Cheng G, Xue Q, Xu H. N/S-Co-doped carbon dot–based FRET ratiometric fluorescence aptasensing platform modulated with entropy-driven DNA amplifier for ochratoxin A detection. Anal Bioanal Chem. 2023;415:4649–60.
Dong X, Ong SY, Zhang C, Chen W, Du S, Xiao Q, Gao L, Yao SQ. Broad-spectrum polymeric nanoquencher as an efficient fluorescence sensing platform for biomolecular detection. ACS Sens. 2021;6:3102–11.
Jia Y, Han J, Wang H, Hong W, Wang H, Zhang M, Li Z. Ultrasensitive quantification of multiplexed mRNA variants via splice-junction anchored DNA probes and SplintR ligase-initiated PCR. Chem Commun. 2021;57:10011–4.
Li H, Warden AR, Su W, He J, Zhi X, Wang K, Zhu L, Shen G, Ding X. Highly sensitive and portable mRNA detection platform for early cancer detection. J Nanobiotechnol. 2021;19:1–10.
He S, Zhao X, Chen F, Chen C, Gong H, Cai C. Detection of long mRNA sequences by a Y-shaped DNA probe with three target-binding segments. Anal Chim Acta. 2023;1277: 341633.
Moon J, Lim J, Lee S, Son HY, Rho HW, Kim H, Kang H, Jeong J, Lim EK, Jung J, Huh YM, Park HG, Kang T. Urinary exosomal mRNA detection using novel isothermal gene amplification method based on three-way junction. Biosens Bioelectron. 2020;167: 112474.
Lin Q, Ye X, Huang Z, Yang B, Fang X, Chen H, Kong J. Graphene oxide-based suppression of nonspecificity in loop-mediated isothermal amplification enabling the sensitive detection of cyclooxygenase-2 mRNA in colorectal cancer. Anal Chem. 2019;91:15694–702.
Yin P, Choi HM, Calvert CR, Pierce NA. Programming biomolecular self-assembly pathways. Nature. 2008;451:318–22.
Zhao Y, Chen F, Li Q, Wang L, Fan C. Isothermal amplification of nucleic acids. Chem Rev. 2015;115:12491–545.
Liu J, Zhang Y, Xie H, Zhao L, Zheng L, Ye H. Applications of catalytic hairpin assembly reaction in biosensing. Small. 2019;15:1902989.
Dong Z, Gao D, Li Y, An K, Ni J, Meng L, Wu H. Self-assembled DNA nanoparticles enable cascade circuits for mRNA detection and imaging in living cells. Anal Chim Acta. 2023;1249: 340934.
Han Y, Hu H, Yu L, Zeng S, Min JZ, Cai S. A duplex-specific nuclease (DSN) and catalytic hairpin assembly (CHA)-mediated dual amplification method for miR-146b detection. Analyst. 2023;148:556–61.
Shin J, Kang N, Kim B, Hong H, Yu L, Kim J, Kang H, Kim JS. One-dimensional nanomaterials for cancer therapy and diagnosis. Chem Soc Rev. 2023;52:4488–514.
Chang M, Dong C, Huang H, Ding L, Feng W, Chen Y. Nanobiomimetic medicine. Adv Funct Mater. 2022;32:2204791.
van der Meel R, Sulheim E, Shi Y, Kiessling F, Mulder WJ, Lammers T. Smart cancer nanomedicine. Nat Nanotechnol. 2019;14:1007–17.
Xu J, Fang L, Shi M, Huang Y, Yao L, Zhao S, Zhang L, Liang H. A peptide-based four-color fluorescent polydopamine nanoprobe for multiplexed sensing and imaging of proteases in living cells. Chem Commun. 2019;55:1651–4.
Mao W, Hu C, Zheng H, Xie J, Shi X, Du Y, Wang F. A functionalized polydopamine theranostic nanoprobe for efficient imaging of miRNA-21 and in vivo synergetic cancer therapy. Mol Ther-Nucl Acids. 2020;22:27–37.
Yao Y, Zhao D, Li N, Shen F, Machuki JOA, Yang D, Li J, Tang D, Yu Y, Tian J, Dong H, Gao F. Multifunctional Fe3O4@polydopamine@DNA-fueled molecular machine for magnetically targeted intracellular Zn2+ imaging and fluorescence/MRI guided photodynamic-photothermal therapy. Anal Chem. 2019;91:7850–7.
Qiu H, Wang J, Zhi Y, Yan B, Huang Y, Li J, Shen C, Dai L, Fang Q, Shi C, Li W. Hyaluronic acid-conjugated fluorescent probe-shielded polydopamine nanomedicines for targeted imaging and chemotherapy of bladder cancer. ACS Appl Mater Interfaces. 2023;15:40.
Sun P, Li Z, Zhang D, Zeng W, Zheng Y, Mei L, Chen H, Gao N, Zeng X. Multifunctional biodegradable nanoplatform based on oxaliplatin prodrug cross-linked mesoporous polydopamine for enhancing cancer synergetic therapy. Chinese Chem Lett. 2023;35: 108346.
Xue C, Li M, Liu C, Li Y, Fei Y, Hu Y, Cai K, Zhao Y, Luo Z. NIR-actuated remote activation of ferroptosis in target tumor cells through a photothermally responsive iron-chelated biopolymer nanoplatform. Angew Chem Int Edit. 2021;60:8938–47.
He P, Han W, Bi C, Song W, Niu S, Zhou H, Zhang X. Many birds, one stone: a smart nanodevice for ratiometric dual-spectrum assay of intracellular microRNA and multimodal synergetic cancer therapy. ACS Nano. 2021;15:6961–76.
Wu Y, Huang Y, Tu C, Wu F, Tong G, Su Y, Xu L, Zhang X, Xiong S, Zhu X. A mesoporous polydopamine nanoparticle enables highly efficient manganese encapsulation for enhanced MRI-guided photothermal therapy. Nanoscale. 2021;13:6439–46.
Wang Y, Pang X, Wang J, Cheng Y, Song Y, Sun Q, You Q, Tan F, Li J, Li N. Magnetically-targeted and near infrared fluorescence/magnetic resonance/photoacoustic imaging-guided combinational anti-tumor phototherapy based on polydopamine-capped magnetic Prussian blue nanoparticles. J Mater Chem B. 2018;6:2460–73.
Ni Z, Zhang D, Zhen S, Liang X, Gong X, Zhao Z, Ding D, Feng G, Tang BZ. NIR light-driven pure organic janus-like nanoparticles for thermophoresis-enhanced photothermal therap. Biomaterials. 2023;301: 122261.
An D, Fu J, Zhang B, Xie N, Nie G, Ågren H, Qiu M, Zhang H. NIR-II responsive inorganic 2D nanomaterials for cancer photothermal therapy: recent advances and future challenges. Adv Funct Mater. 2021;31:2101625.
Cao H, Yang Y, Liang M, Ma Y, Sun N, Gao X, Li J. Pt@polydopamine nanoparticles as nanozymes for enhanced photodynamic and photothermal therapy. Chem Commun. 2021;57:255–8.
Song H, Yu M, Lu Y, Gu Z, Yang Y, Zhang M, Fu Z, Yu C. Plasmid DNA delivery: nanotopography matters. J Am Chem Soc. 2017;139:18247–54.
Liu L, Yang Z, Liu C, Wang M, Chen X. Preparation of PEI-modified nanoparticles by dopamine self-polymerization for efficient DNA delivery. Biotechnol Appl Bioc. 2023;70:824–34.
Dai G, Choi CKK, Choi CHJ, Fong WP, Ng DK. Glutathione-degradable polydopamine nanoparticles as a versatile platform for fabrication of advanced photosensitisers for anticancer therapy. Biomater Sci-Uk. 2022;10:189–201.
Qiang W, Li W, Li X, Chen X, Xu D. Bioinspired polydopamine nanospheres: a superquencher for fluorescence sensing of biomolecules. Chem Sci. 2014;5:3018–24.
Funding
This work was supported by the National Natural Science Foundations of China (22064002, 22364021), the Natural Science Foundation of Guangxi Province (2020GXNSFAA159072), the PhD Research Startup Program of Yulin Normal University (G2023Zk04), and the Guangxi University Students Innovative Project (S202310606116).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, J., Zhong, X., Fan, M. et al. Enhancing intracellular mRNA precise imaging-guided photothermal therapy with a nucleic acid-based polydopamine nanoprobe. Anal Bioanal Chem 416, 849–859 (2024). https://doi.org/10.1007/s00216-023-05062-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-023-05062-2