Abstract
Testosterone (TTe) and free testosterone (FTe) are clinically important indicators for the diagnosis of androgen disorders, so accurate quantitative determination of them in serum is clinically of paramount significance. Currently, there is no available method suitable for routine and simultaneous measurement of TTe and FTe. Here, we developed a new UPLC-MS/MS method to quantify serum TTe and FTe simultaneously and accurately. Rapid equilibrium dialysis was used to obtain FTe in serum followed by derivatization with hydroxylamine hydrochloride. With these strategies, TTe and FTe could be measured in single injection. After optimizing the extraction and derivatization conditions, the performance of LC–MS/MS was evaluated and applied to quantify the levels of TTe and FTe in clinical samples from 42 patients. The assays were linear for TTe within the range of 0.2–30 ng/mL and for FTe within the range of 1.5–1000 pg/mL. This improved method provided a limit of quantification for TTe of 0.2 ng/mL and for FTe of 1.5 pg/mL. The intra- and inter-run CVs were less than 4.3% and 3.6% for TTe and less than 8.2% and 6.7% for FTe, respectively. The intra- and inter-run accuracies for both TTe and FTe were in the range of 96.1–108.1%. Interference, carryover effect, and matrix effect were in acceptable range. In conclusion, our new LC–MS/MS method is simple to perform and can serve as a reliable method for simultaneous determination of TTe and FTe in clinical practice, providing important information for diagnosis, treatment, and monitoring of androgen-related diseases.
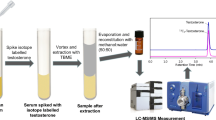
Graphical Abstract







Similar content being viewed by others
References
Van Uytfanghe K, Stöckl D, Kaufman JM, Fiers T, Ross HA, De Leenheer AP, Thienpont LM. Evaluation of a candidate reference measurement procedure for serum free testosterone based on ultrafiltration and isotope dilution–gas chromatography–mass spectrometry. Clin Chem. 2004;50(11):2101-2110. https://doi.org/10.1373/clinchem.2004.037358.
Basaria S. Male hypogonadism. The Lancet. 2014;383(9924):1250–63. https://doi.org/10.1016/S0140-6736(13)61126-5.
Handelsman DJ, Hirschberg AL, Bermon S. Circulating testosterone as the hormonal basis of sex differences in athletic performance. Endocr Rev. 2018;39(5):803–29. https://doi.org/10.1210/er.2018-00020.
Mirand EA, Gordon AS, Wenig J. Mechanism of testosterone action in erythropoiesis. Nature. 1965;206(4981):270–2.
Agretti P, Pelosini C, Bianchi L, Grosso AD, Saba A, Canale D, Sessa MR. Importance of total and measured free testosterone in diagnosis of male hypogonadism: immunoassay versus mass spectrometry in a population of healthy young/middle-aged blood donors. J Endocrinol Invest. 2021;44(2):321–6. https://doi.org/10.1007/s40618-020-01304-7.
Salameh WA, Redor-Goldman MM, Clarke NJ, Reitz RE, Caulfield MP. Validation of a total testosterone assay using high-turbulence liquid chromatography tandem mass spectrometry: total and free testosterone reference ranges. Steroids. 2010;75(2):169–75. https://doi.org/10.1016/j.steroids.2009.11.004.
Rhea JM, French D, Molinaro RJ. Direct total and free testosterone measurement by liquid chromatography tandem mass spectrometry across two different platforms. Clin Biochem. 2013;46(7):656–64. https://doi.org/10.1016/j.clinbiochem.2013.01.005.
Kanakis GA, Tsametis CP, Goulis DG. Measuring testosterone in women and men. Maturitas. 2019;125:41–4. https://doi.org/10.1016/j.maturitas.2019.04.203.
Goldman AL, Bhasin S, Wu FCW, Krishna M, Matsumoto AM, Jasuja R. A reappraisal of testosterone’s binding in circulation: physiological and clinical implications. Endocr Rev. 2017;38(4):302–24. https://doi.org/10.1210/er.2017-00025.
Grassi G, Polledri E, Fustinoni S, Chiodini I, Ceriotti F, D'Agostino S, Filippi F, Somigliana E, Mantovani G, Arosio M, Morelli V. Hyperandrogenism by liquid chromatography tandem mass spectrometry in PCOS: focus on testosterone and androstenedione. J Clin Med. 2020;10(1). https://doi.org/10.3390/jcm10010119.
Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocr Metab. 2010;95(6):2536–59. https://doi.org/10.1210/jc.2009-2354.
Huhtaniemi IT, Tajar A, Lee DM, O’Neill TW, Finn JD, Bartfai G, Boonen S, Casanueva FF, Giwercman A, Han TS, Kula K, Labrie F, Lean MEJ, Pendleton N, Punab M, Silman AJ, Vanderschueren D, Forti G, Wu FCW. Comparison of serum testosterone and estradiol measurements in 3174 European men using platform immunoassay and mass spectrometry; relevance for the diagnostics in aging men. Eur J Endocrinol. 2012;166(6):983–91. https://doi.org/10.1530/EJE-11-1051.
Guzelce EC, Galbiati F, Goldman AL, Gattu AK, Basaria S, Bhasin S. Accurate measurement of total and free testosterone levels for the diagnosis of androgen disorders. Best Pract Res Cl En. 2022;36(4):101683. https://doi.org/10.1016/j.beem.2022.101683.
Welker KM, Lassetter B, Brandes CM, Prasad S, Koop DR, Mehta PH. A comparison of salivary testosterone measurement using immunoassays and tandem mass spectrometry. Psychoneuroendocrino. 2016;71:180–8. https://doi.org/10.1016/j.psyneuen.2016.05.022.
Moal V, Mathieu E, Reynier P, Malthièry Y, Gallois Y. Low serum testosterone assayed by liquid chromatography-tandem mass spectrometry. Comparison with five immunoassay techniques. Clin Chim Acta. 2007;386(1):12–19. https://doi.org/10.1016/j.cca.2007.07.013.
La’ulu SL, Kalp KJ, Straseski JA. How low can you go? Analytical performance of five automated testosterone immunoassays. Clin Biochem. 2018;58:64–71. https://doi.org/10.1016/j.clinbiochem.2018.05.008.
de Ronde W, van der Schouw YT, Pols HAP, Gooren LJG, Muller M, Grobbee DE, de Jong FH. Calculation of bioavailable and free testosterone in men: a comparison of 5 published algorithms. Clin Chem. 2006;52(9):1777–84. https://doi.org/10.1373/clinchem.2005.063354.
Van Uytfanghe K, Stöckl D, Kaufman JM, Fiers T, De Leenheer A, Thienpont LM. Validation of 5 routine assays for serum free testosterone with a candidate reference measurement procedure based on ultrafiltration and isotope dilution–gas chromatography–mass spectrometry. Clin Biochem. 2005;38(3):253–61. https://doi.org/10.1016/j.clinbiochem.2004.12.001.
Raverot V, Lopez J, Grenot C, Pugeat M, Déchaud H. New approach for measurement of non-SHBG-bound testosterone in human plasma. Anal Chim Acta. 2010;658(1):87–90. https://doi.org/10.1016/j.aca.2009.10.057.
Seger C, Salzmann L. After another decade: LC–MS/MS became routine in clinical diagnostics. Clin Biochem. 2020;82:2–11. https://doi.org/10.1016/j.clinbiochem.2020.03.004.
Desai R, Harwood DT, Handelsman DJ. Simultaneous measurement of 18 steroids in human and mouse serum by liquid chromatography–mass spectrometry without derivatization to profile the classical and alternate pathways of androgen synthesis and metabolism. Clin Mass Spectrom. 2019;11:42–51. https://doi.org/10.1016/j.clinms.2018.12.003.
Liu D, Zhao R, Zhao S, Wang Z, Liu R, Wang F, Gao Y. A developed HPLC-MS/MS method to quantitate 5 steriod hormones in clinical human serum by using PBS as the surrogate matrix. J Chromatogr B. 2021;1186:123002. https://doi.org/10.1016/j.jchromb.2021.123002.
Chen F, Cheng Z, Wang Z, Peng Y, Wang B, Guo W, Pan B. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) based assay for the simultaneous quantification of 18-hydroxycorticosterone, 18-hydroxycortisol and 18-oxocortisol in human plasma. J Chromatogr B. 2022;1188:123030. https://doi.org/10.1016/j.jchromb.2021.123030.
Gravitte A, Archibald T, Cobble A, Kennard B, Brown S. Liquid chromatography–mass spectrometry applications for quantification of endogenous sex hormones. Biomed Chromatogr. 2021;35(1):e5036. https://doi.org/10.1002/bmc.5036.
Yuan T-F, Le J, Cui Y, Peng R, Wang S-T, Li Y. An LC-MS/MS analysis for seven sex hormones in serum. J Pharmaceut Biomed. 2019;162:34–40. https://doi.org/10.1016/j.jpba.2018.09.014.
Yu S, Zou Y, Yin Y, Yu J, Li Q, Xie S, Luo W, Ma X, Wang D, Qiu L. Establishing and verifying a robust liquid chromatography-tandem mass spectrometry method to simultaneously measure seven androgens present in plasma samples. Separations. 2022;9(11). https://doi.org/10.3390/separations9110377.
Chen Y, Yazdanpanah M, Wang XY, Hoffman BR, Diamandis EP, Wong PY. Direct measurement of serum free testosterone by ultrafiltration followed by liquid chromatography tandem mass spectrometry. Clin Biochem. 2010;43(4–5):490–6. https://doi.org/10.1016/j.clinbiochem.2009.12.005.
Star-Weinstock M, Williamson BL, Dey S, Pillai S, Purkayastha S. LC-ESI-MS/MS analysis of testosterone at sub-picogram levels using a novel derivatization reagent. Anal Chem. 2012;84(21):9310–7. https://doi.org/10.1021/ac302036r.
Colletti JD, Redor-Goldman MM, Pomperada AE, Ghoshal AK, Wu WW, McPhaul MJ, Clarke NJ. Sample multiplexing: increased throughput for quantification of total testosterone in serum by liquid chromatography-tandem mass spectrometry. Clin Chem. 2020;66(9):1181–9. https://doi.org/10.1093/clinchem/hvaa117.
Zhang X, Xu H, Zhou C, Yang L, Zhai S, Yang P, Zhao R, Li R. Magnetic solid phase extraction followed by in-situ derivatization with core–shell structured magnetic graphene oxide nanocomposite for the accurate quantification of free testosterone and free androstenedione in human serum. J Chromatogr B. 2022;1196:123188. https://doi.org/10.1016/j.jchromb.2022.123188.
Lie M, Thorstensen K. A precise, sensitive and stable LC-MSMS method for detection of picomolar levels of serum aldosterone. Scand J Clin Lab Inv. 2018;78(5):379–85. https://doi.org/10.1080/00365513.2018.1480060.
Chen F, Cheng Z, Peng Y, Wang Z, Huang C, Liu D, Wang B, Pan B, Guo W. A liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based assay for simultaneous quantification of aldosterone, renin activity, and angiotensin II in human plasma. J Chromatogr B. 2021;1179:122740. https://doi.org/10.1016/j.jchromb.2021.122740.
He B, Di X, Guled F, Harder AVE, van den Maagdenberg AMJM, Terwindt GM, Krekels EHJ, Kohler I, Harms A, Ramautar R, Hankemeier T. Quantification of endocannabinoids in human cerebrospinal fluid using a novel micro-flow liquid chromatography-mass spectrometry method. Anal Chim Acta. 2022;1210:339888. https://doi.org/10.1016/j.aca.2022.339888.
Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, Snyder PJ, Swerdloff RS, Wu FC, Yialamas MA. Testosterone therapy in men with hypogonadism: an Endocrine Society* clinical practice guideline. J Clin Endocr Metab. 2018;103(5):1715–44. https://doi.org/10.1210/jc.2018-00229.
Li Y, Zhai Y, Li L, Lu Y, Su S, Liu Y, Xu Z, Xin M, Zhang Q, Cao Z. Divergent associations between serum androgens and ovarian reserve markers revealed in patients with polycystic ovary syndrome. Front Endocrinol. 2022;13:881740. https://doi.org/10.3389/fendo.2022.881740.
Cao Z, Lu Y, Cong Y, Liu Y, Li Y, Wang H, Zhang Q, Huang W, Liu J, Dong Y, Tang G, Luo YR, Yin C, Zhai Y. Simultaneous quantitation of four androgens and 17-hydroxyprogesterone in polycystic ovarian syndrome patients by LC-MS/MS. J Clin Lab Anal. 2020;34(12):e23539. https://doi.org/10.1002/jcla.23539.
de Boer T, Meijering H. Equilibrium dialysis, ultracentrifugation, and ultrafiltration in LC-MS bioanalysis. In: Sample preparation in LC-MS bioanalysis. 2019; pp 45–51. https://doi.org/10.1002/9781119274315.ch3.
Schuijt MP, Sweep CGJ, van der Steen R, Olthaar AJ, Stikkelbroeck NMML, Ross HA, van Herwaarden AE. Validity of free testosterone calculation in pregnant women. Endocr connect. 2019;8(6):672–9. https://doi.org/10.1530/EC-19-0110.
Antonio L, Pauwels S, Laurent MR, Vanschoubroeck D, Jans I, Billen J, Claessens F, Decallonne B, De Neubourg D, Vermeersch P, Vanderschueren D. Free testosterone reflects metabolic as well as ovarian disturbances in subfertile oligomenorrheic women. Int J Endocrinol. 2018;2018:7956951–7956951. https://doi.org/10.1155/2018/7956951.
Waters NJ, Jones R, Williams G, Sohal B. Validation of a rapid equilibrium dialysis approach for the measurement of plasma protein binding. J Pharm Sci. 2008;97(10):4586–95. https://doi.org/10.1002/jps.21317.
Funding
Financial support was provided by the National Natural Science Foundation of China (Grant No. 22004086), Shenzhen Science and Technology Innovation Commission (Grant No. JCYJ20210324115601005), and the Guangdong Province Basic and Applied Basic Research Regional Joint Fund-Youth Fund Project (Grant No. 2021A1515110849).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
The study was approved by the Ethics Committee of Shenzhen Second People’s Hospital.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, R., Hong, Y., Wu, Y. et al. Simultaneous quantification of total and free testosterone in human serum by LC–MS/MS. Anal Bioanal Chem 415, 6851–6861 (2023). https://doi.org/10.1007/s00216-023-04963-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-023-04963-6