Abstract
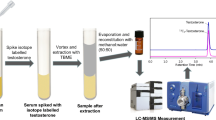
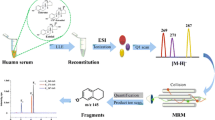
Reliable measurement of total testosterone and estradiol is critical for their use as biomarkers of hormone-related disorders in patient care and translational research. We developed and validated a mass spectrometry method to simultaneously quantify these analytes in human serum without chemical derivatization. Serum is equilibrated with isotopic internal standards and treated with acidic buffer to release hormones from their binding proteins. Lipids are isolated and polar impurities are removed by two serial liquid–liquid extraction steps. Total testosterone and estradiol are measured using liquid chromatography tandem mass spectrometry (LC–MS/MS) in combination of positive and negative electrospray ionization modes. The method shows broad analytical measurement range for both testosterone 0.03–48.5 nM (0.75–1400 ng/dL) and estradiol 11.0–5138 pM (2.99–1400 pg/mL) and excellent agreement with certified reference materials (mean bias less than 2.1% to SRM 971, BCR 576, 577, and 578) and a high order reference method (mean bias 1.25% for testosterone and −0.84% for estradiol). The high accuracy of the method was monitored and certified by CDC Hormone Standardization (HoSt) Program for 2 years with mean bias −0.7% (95% CI −1.6% to 0.2%) for testosterone and 0.1% (95% CI −2.2% to 2.3%) for estradiol. The method precision over a 2-year period for quality control pools at low, medium, and high concentrations was 2.7–2.9% for testosterone and 3.3–5.3% for estradiol. With the consistently excellent accuracy and precision, this method is readily applicable for high-throughput clinical and epidemiological studies.


Similar content being viewed by others
References
Sizonenko PC. Normal sexual maturation. Pediatrician. 1987;14(4):191–201.
Grossmann M. Testosterone and glucose metabolism in men: current concepts and controversies. J Endocrinol. 2014;220(3):R37–55.
Khera M. Male hormones and men's quality of life. Curr Opin Urol. 2016;26(2):152–7.
Cauley JA. Estrogen and bone health in men and women. Steroids. 2015;99(Pt A):11–5.
Mongraw-Chaffin ML, Anderson CA, Allison MA, Ouyang P, Szklo M, Vaidya D, et al. Association between sex hormones and adiposity: qualitative differences in women and men in the multi-ethnic study of atherosclerosis. J Clin Endocrinol Metab. 2015;100(4):E596–600.
Loomba-Albrecht LA, Styne DM. The physiology of puberty and its disorders. Pediatr Ann. 2012;41(4):e1–9.
Rahhal SN, Fuqua JS, Lee PA. The impact of assay sensitivity in the assessment of diseases and disorders in children. Steroids. 2008;73(13):1322–7.
Khera M, Adaikan G, Buvat J, Carrier S, El-Meliegy A, Hatzimouratidis K, et al. Diagnosis and treatment of testosterone deficiency: recommendations from the fourth international consultation for sexual medicine (ICSM 2015). J Sex Med. 2016;13(12):1787–804.
Schulman CC, Irani J, Morote J, Schalken JA, Montorsi F, Chlosta PL, et al. Testosterone measurement in patients with prostate cancer. Eur Urol. 2010;58(1):65–74.
Peavey M, Akbas N, Gibbons W, Zarutskie P, Devaraj S. ANNALS EXPRESS: optimization of estradiol assays to improve utility in an in vitro fertilization setting. Ann Clin Biochem. 2017:4563217691788.
Lonning PE. Estradiol measurement in translational studies of breast cancer. Steroids. 2015;99(Pt A):26–31.
Demers LM. Testosterone and estradiol assays: current and future trends. Steroids. 2008;73(13):1333–8.
Herati AS, Cengiz C, Lamb DJ. Assays of serum testosterone. Urol Clin North Am. 2016;43(2):177–84.
Santen RJ, Lee JS, Wang S, Demers LM, Mauras N, Wang H, et al. Potential role of ultra-sensitive estradiol assays in estimating the risk of breast cancer and fractures. Steroids. 2008;73(13):1318–21.
Cawood ML, Field HP, Ford CG, Gillingwater S, Kicman A, Cowan D, et al. Testosterone measurement by isotope-dilution liquid chromatography-tandem mass spectrometry: validation of a method for routine clinical practice. Clin Chem. 2005;51(8):1472–9.
Wang C, Catlin DH, Demers LM, Starcevic B, Swerdloff RS. Measurement of total serum testosterone in adult men: comparison of current laboratory methods versus liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab. 2004;89(2):534–43.
Kushnir MM, Rockwood AL, Roberts WL, Pattison EG, Bunker AM, Fitzgerald RL, et al. Performance characteristics of a novel tandem mass spectrometry assay for serum testosterone. Clin Chem. 2006;52(1):120–8.
Rosner W, Hankinson SE, Sluss PM, Vesper HW, Wierman ME. Challenges to the measurement of estradiol: an endocrine society position statement. J Clin Endocrinol Metab. 2013;98(4):1376–87.
Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Position statement: utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab. 2007;92(2):405–13.
Demers LM, Hankinson SE, Haymond S, Key T, Rosner W, Santen RJ, et al. Measuring estrogen exposure and metabolism: workshop recommendations on clinical issues. J Clin Endocrinol Metab. 2015;100(6):2165–70.
Wang Y, Gay GD, Botelho JC, Caudill SP, Vesper HW. Total testosterone quantitative measurement in serum by LC–MS/MS. Clin Chim Acta. 2014;436:263–7.
Caudill SP, Schleicher RL, Pirkle JL. Multi-rule quality control for the age-related eye disease study. Stat Med. 2008;27(20):4094–106.
CLSI. C62-A: liquid chromatography-mass spectrometry methods; approved guideline-first edition. Wayne: Clinical and Laboraotory Standards Institue; 2014.
Becker D, Christensen R, Currie L, Diamondstone B, Eberhardt K, Gills T, et al. Use of NIST standard reference materials for decisions on performance of analytical chemical methods and laboratories Gaithersburg, MD: NIST Special Publication 829; 1992.
CLSI. EP9-A2: method comparison and bias estimation using patient samples; approved guidline-second edition. Wayne: Clinical and Laboratory Standards Institute; 2002.
Tai SS, Xu B, Welch MJ, Phinney KW. Development and evaluation of a candidate reference measurement procedure for the determination of testosterone in human serum using isotope dilution liquid chromatography/tandem mass spectrometry. Anal Bioanal Chem. 2007;388(5–6):1087–94.
Thienpont LM, De Leenheer AP. Efforts by industry toward standardization of serum estradiol-17 beta measurements. Clin Chem. 1998;44(3):671–4.
Thienpont LM, De Brabandere VI, Stockl D, De Leenheer AP. Use of cyclodextrins for prepurification of progesterone and testosterone from human serum prior to determination with isotope dilution gas chromatography/mass spectrometry. Anal Chem. 1994;66(22):4116–9.
Botelho JC, Ribera A, Cooper HC, Vesper HW. Evaluation of an isotope dilution HPLC tandem mass spectrometry candidate reference measurement procedure for total 17-beta estradiol in human serum. Anal Chem. 2016;88(22):11123–9.
Botelho JC, Shacklady C, Cooper HC, Tai SS, Van Uytfanghe K, Thienpont LM, et al. Isotope-dilution liquid chromatography-tandem mass spectrometry candidate reference method for total testosterone in human serum. Clin Chem. 2013;59(2):372–80.
Yun YM, Botelho JC, Chandler DW, Katayev A, Roberts WL, Stanczyk FZ, et al. Performance criteria for testosterone measurements based on biological variation in adult males: recommendations from the Partnership for the Accurate Testing of Hormones. Clin Chem. 2012;58(12):1703–10.
Vesper HW, Botelho JC, Vidal ML, Rahmani Y, Thienpont LM, Caudill SP. High variability in serum estradiol measurements in men and women. Steroids. 2014;82:7–13.
CLSI. EP5-A3: evaluation of precision of quantitative measurement procedures; approved guideline-third edition. Wayne: Clinical and Laboratory Standards Institute; 2014.
Rodriguez M, Orescan DB. Confirmation and quantitation of selected sulfonylurea, imidazolinone, and sulfonamide herbicides in surface water using electrospray LC/MS. Anal Chem. 1998;70(13):2710–7.
CLSI. EP17-A2: evaluation of detection capability for clinical laboratory measurement procedures; approved guideline-second edition. Wayne: Clinical and Laboratory Standards Institute; 2012.
Taylor JK. Quality assurance of chemical measurements. Boca Raton: CRC; 1987.
Fraser CG. Biological variation: from principles to practice. Washington, DC: American Association for Clinical Chemistry; 2001.
Ricos C, Alvarez V, Cava F, Garcia-Lario JV, Hernandez A, Jimenez CV, et al. Current databases on biological variation: pros, cons and progress. Scand J Clin Lab Invest. 1999;59(7):491–500.
Vesper HW, Bhasin S, Wang C, Tai SS, Dodge LA, Singh RJ, et al. Interlaboratory comparison study of serum total testosterone [corrected] measurements performed by mass spectrometry methods. Steroids. 2009;74(6):498–503.
Nelson RE, Grebe SK, DJ OK, Singh RJ. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem. 2004;50(2):373–84.
Kushnir MM, Rockwood AL, Bergquist J, Varshavsky M, Roberts WL, Yue B, et al. High-sensitivity tandem mass spectrometry assay for serum estrone and estradiol. Am J Clin Pathol. 2008;129(4):530–9.
Schofield RC, Mendu DR, Ramanathan LV, Pessin MS, Carlow DC. Sensitive simultaneous quantitation of testosterone and estradiol in serum by LC–MS/MS without derivatization and comparison with the CDC HoSt program. J Chromatogr B. 2017;1048:70–6.
Zhang Q, Han L, Wang J, Lin H, Ke P, Zhuang J, et al. Simultaneous quantitation of endogenous estrone, 17beta-estradiol, and estriol in human serum by isotope-dilution liquid chromatography-tandem mass spectrometry for clinical laboratory applications. Anal Bioanal Chem. 2017;409(10):2627–38.
Vesper HW, Botelho JC. Standardization of testosterone measurements in humans. J Steroid Biochem Mol Biol. 2010;121(3–5):513–9.
Hurwitz AR, Liu ST. Determination of aqueous solubility and pKa values of estrogens. J Pharm Sci. 1977;66(5):624–7.
Boggs AS, Bowden JA, Galligan TM, Guillette Jr LJ, Kucklick JR. Development of a multi-class steroid hormone screening method using Liquid Chromatography/Tandem Mass Spectrometry (LC–MS/MS). Anal Bioanal Chem. 2016;408(15):4179–90.
Ray JA, Kushnir MM, Rockwood AL, Meikle AW. Direct measurement of free estradiol in human serum and plasma by equilibrium dialysis-liquid chromatography-tandem mass spectrometry. Methods Mol Biol. 2016;1378:99–108.
Vitku J, Chlupacova T, Sosvorova L, Hampl R, Hill M, Heracek J, et al. Development and validation of LC–MS/MS method for quantification of bisphenol A and estrogens in human plasma and seminal fluid. Talanta. 2015;140:62–7.
Keski-Rahkonen P, Desai R, Jimenez M, Harwood DT, Handelsman DJ. Measurement of estradiol in human serum by LC–MS/MS using a novel estrogen-specific derivatization reagent. Anal Chem. 2015;87(14):7180–6.
Acknowledgements
We would like to acknowledge CDC Hormone Laboratory members Lumi Duke, Paul Kim, Gabrielle D. Gay, Krista Poynterk, and Otoe Sugahara for their support in the laboratory measurements. We thank Brandon Laughlin and Dr. Uliana Danilenko for their edits and review (all with the National Center for Environmental Health, Centers for Disease Control and Prevention, Atlanta, Georgia).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclaimer
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhou, H., Wang, Y., Gatcombe, M. et al. Simultaneous measurement of total estradiol and testosterone in human serum by isotope dilution liquid chromatography tandem mass spectrometry. Anal Bioanal Chem 409, 5943–5954 (2017). https://doi.org/10.1007/s00216-017-0529-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0529-x