Abstract
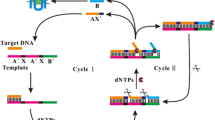
The phosphorylation process of DNA by T4 polynucleotide kinase (T4 PNK) plays a crucial role in DNA recombination, DNA replication, and DNA repair. Traditional monomeric G-quadruplex (G4) systems are always activated by single cation such as K+ or Na+. The conformation transformation caused by the coexistence of multiple cations may interfere with the signal readout and limit their applications in physiological system. In view of the stability of dimeric G4 in multiple cation solution, we reported a label-free T4 PNK fluorescence sensor based on split dimeric G4 and ligation-induced dimeric G4/thioflavin T (ThT) conformation. The dimeric G4 was divided into two independent pieces of one normal monomeric G4 and the other monomeric G4 fragment phosphorylated by T4 PNK in order to decrease the background signal. With the introduction of template DNA, DNA ligase, and invasive DNA, the dimeric G4 could be generated and liberated to combine with ThT to show obvious fluorescence signal. Using our strategy, the linear range from 0.005 to 0.5 U mL−1, and the detection limit of 0.0021 U mL−1 could be achieved without the consideration of interference caused by the coexistence of multiple cations. Additionally, research in real sample determination and inhibition effect investigations indicated its further potential application value in biochemical process research and clinic diagnostics.
Graphical abstract







Similar content being viewed by others
References
Richardson CC. Phosphorylation of nucleic acid by an enzyme from T4 bacteriophage-infected Escherichia coli. Proc Natl Acad Sci U S A. 1965;54(1):158–65. https://doi.org/10.1073/pnas.54.1.158.
Novogrodsky A, Hurwitz J. The enzymatic phosphorylation of ribonucleic acid and deoxyribonucleic acid. I. Phosphorylation at 5’-hydroxyl termini. J Biol Chem. 1966;241(12):2923–32. https://doi.org/10.1016/S0021-9258(18)96553-1.
Ma C, Yeung ES. Highly sensitive detection of DNA phosphorylation by counting single nanoparticles. Anal Bioanal Chem. 2010;397(6):2279–84. https://doi.org/10.1007/s00216-010-3801-x.
Karimi-Busheri F, Daly G, Robins P, Canas B, Pappin DJ, Sgouros J, Miller GG, Fakhrai H, Davis EM, Le Beau MM, Weinfeld M. Molecular characterization of a human DNA kinase. J Biol Chem. 1999;274(34):24187–94. https://doi.org/10.1074/jbc.274.34.24187.
Chalasani SL, Kawale AS, Akopiants K, Yu Y, Fanta M, Weinfeld M, Povirk LF. Persistent 3’-phosphate termini and increased cytotoxicity of radiomimetic DNA double-strand breaks in cells lacking polynucleotide kinase/phosphatase despite presence of an alternative 3’-phosphatase. DNA Repair (Amst). 2018;68:12–24. https://doi.org/10.1016/j.dnarep.2018.05.002.
Tang Z, Wang K, Tan W, Ma C, Li J, Liu L, Guo Q, Meng X. Real-time investigation of nucleic acids phosphorylation process using molecular beacons. Nucleic Acids Res. 2005;33(11): e97. https://doi.org/10.1093/nar/gni096.
Sharma S, Doherty KM, Brosh RJ. Mechanisms of RecQ helicases in pathways of DNA metabolism and maintenance of genomic stability. Biochem J. 2006;398(3):319–37. https://doi.org/10.1042/BJ20060450.
Tahbaz N, Subedi S, Weinfeld M. Role of polynucleotide kinase/phosphatase in mitochondrial DNA repair. Nucleic Acids Res. 2012;40(8):3484–95. https://doi.org/10.1093/nar/gkr1245.
Ahel I, Rass U, El-Khamisy SF, Katyal S, Clements PM, Mckinnon PJ, Caldecott KW, West SC. The neurodegenerative disease protein aprataxin resolves abortive DNA ligation intermediates. Nature. 2006;443(7112):713–6. https://doi.org/10.1038/nature05164.
Aggarwal M, Banerjee T, Sommers JA, Iannascoli C, Pichierri P, Shoemaker RH, Brosh RM. Werner syndrome helicase has a critical role in DNA damage responses in the absence of a functional Fanconi anemia pathway. Cancer Res. 2013;73(17):5497–507. https://doi.org/10.1158/0008-5472.CAN-12-2975.
Amitsur M, Levitz R, Kaufmann G. Bacteriophage T4 anticodon nuclease, polynucleotide kinase and RNA ligase reprocess the host lysine tRNA. Embo J. 1987;6(8):2499–503. https://doi.org/10.1002/j.1460-2075.1987.tb02532.x.
Chappell C, Hanakahi LA, Karimi-Busheri F, Weinfeld M, West SC. Involvement of human polynucleotide kinase in double-strand break repair by non-homologous end joining. Embo J. 2002;21(11):2827–32. https://doi.org/10.1093/emboj/21.11.2827.
Phillips DH, Arlt VM. The 32P-postlabeling assay for DNA adducts. Nat Protoc. 2007;2(11):2772–81. https://doi.org/10.1038/nprot.2007.394.
Wang LK, Shuman S. Domain structure and mutational analysis of T4 polynucleotide kinase. J Biol Chem. 2001;276(29):26868–74. https://doi.org/10.1074/jbc.M103663200.
Whitehouse CJ, Taylor RM, Thistlethwaite A, Zhang H, Karimi-Busheri F, Lasko DD, Weinfeld M, Caldecott KW. XRCC1 stimulates human polynucleotide kinase activity at damaged DNA termini and accelerates DNA single-strand break repair. Cell. 2001;104(1):107–17. https://doi.org/10.1016/s0092-8674(01)00195-7.
Cen Y, Deng WJ, Yu RQ, Chu X. Sensitive fluorescence sensing of T4 polynucleotide kinase activity and inhibition based on DNA/polydopamine nanospheres platform. Talanta. 2018;180:271–6. https://doi.org/10.1016/j.talanta.2017.12.038.
Chen F, Zhao Y, Qi L, Fan C. One-step highly sensitive florescence detection of T4 polynucleotide kinase activity and biological small molecules by ligation-nicking coupled reaction-mediated signal amplification. Biosen Bioelectron. 2013;47:218–24. https://doi.org/10.1016/j.bios.2013.03.034.
Gao M, Guo J, Song Y, Zhu Z, Yang CJ. Detection of T4 polynucleotide kinase via allosteric aptamer probe platform. ACS Appl Mater Interfaces. 2017;9(44):38356–63. https://doi.org/10.1021/acsami.7b14185.
Hou T, Wang X, Liu X, Lu T, Liu S, Li F. Amplified detection of T4 polynucleotide kinase activity by the coupled lambda exonuclease cleavage reaction and catalytic assembly of bimolecular beacons. Anal Chem. 2014;86(1):884–90. https://doi.org/10.1021/ac403458b.
Liu S, Ming J, Lin Y, Wang C, Liu T, Cheng C, Li F. Amplified detection of T4 polynucleotide kinase activity based on a λ-exonuclease cleavage-induced DNAzyme releasing strategy. Sensors Actuators B Chem. 2014;192:157–63. https://doi.org/10.1016/j.snb.2013.10.101.
Zhang X, Zheng C, Ding L, Wu Y, Xu H, Sun Y, Zeng Y, Liu X, Liu J. CRISPR-Cas12a coupled with terminal deoxynucleotidyl transferase mediated isothermal amplification for sensitive detection of polynucleotide kinase activity. Sensors Actuators B Chem. 2021;330: 129317. https://doi.org/10.1016/j.snb.2020.129317.
Zhou H, Tong C, Zou W, Yang Y, Liu Y, Li B, Qin Y, Dang W, Liu B, Wang W. A novel fluorescence method for activity assay and drug screening of T4 PNK by coupling rGO with ligase reaction. Analyst. 2019;144(4):1187–96. https://doi.org/10.1039/c8an02147c.
Zhu Z, Yu R, Chu X. Amplified fluorescence detection of T4 polynucleotide kinase activity and inhibition via a coupled λ exonuclease reaction and exonuclease III-aided trigger DNA recycling. Anal Methods. 2014;6(15):6009–14. https://doi.org/10.1039/C4AY01097C.
Shen X, Ge J, Chen J, Shen Y, Meng H, Li Z, Qu L. A novel fluorescence method for the highly sensitive detection of T4 polynucleotide kinase based on polydopamine nanotubes. New J Chem. 2019;43:16753–8. https://doi.org/10.1039/C9NJ04381K.
Luo R, Zhou H, Dang W, Long Y, Tong C, Xie Q, Daniyal M, Liu B, Wang W. A DNAzyme-rGO coupled fluorescence assay for T4PNK activity in vitro and intracellular imaging. Sensors Actuators B Chem. 2020;310: 127884. https://doi.org/10.1016/j.snb.2020.127884.
Chen X, Cao G, Zhang J, Deng Y, Luo X, Yang M, Huo D, Hou C. An ultrasensitive and point-of-care strategy for enzymes activity detection based on enzyme extends activators to unlock the ssDNase activity of CRISPR/Cas12a (EdU-CRISPR/Cas12a). Sensors Actuators B Chem. 2021;333: 129553. https://doi.org/10.1016/j.snb.2021.129553.
Liu J, Liu Y, Zhang L, Fu S, Su X. Ultra-specific fluorescence detection of DNA modifying enzymes by dissipation system. Biosens Bioelectron. 2022;215: 114561. https://doi.org/10.1016/j.bios.2022.114561.
Shang J, Yu S, Chen Y, Gao Y, Hong C, Li F, Wang F. Real-time investigation of intracellular polynucleotide kinase using a cascaded amplification circuit. Anal Chem. 2021;93(46):15559–66. https://doi.org/10.1021/acs.analchem.1c04033.
Jiang HX, Kong DM, Shen HX. Amplified detection of DNA ligase and polynucleotide kinase/phosphatase on the basis of enrichment of catalytic G-quadruplex DNAzyme by rolling circle amplification. Biosens Bioelectron. 2014;55:133–8. https://doi.org/10.1016/j.bios.2013.12.001.
Liu H, Ma C, Wang J, Chen H, Wang K. Label-free colorimetric assay for T4 polynucleotide kinase/phosphatase activity and its inhibitors based on G-quadruplex/hemin DNAzyme. Anal Biochem. 2017;517:18–21. https://doi.org/10.1016/j.ab.2016.10.022.
Jiang C, Yan C, Jiang J, Yu R. Colorimetric assay for T4 polynucleotide kinase activity based on the horseradish peroxidase-mimicking DNAzyme combined with λ exonuclease cleavage. Anal Chim Acta. 2013;766:88–93. https://doi.org/10.1016/j.aca.2012.12.034.
Jiang Y, Cui J, Zhang T, Wang M, Zhu G, Miao P. Electrochemical detection of T4 polynucleotide kinase based on target-assisted ligation reaction coupled with silver nanoparticles. Anal Chim Acta. 2019;1085:85–90. https://doi.org/10.1016/j.aca.2019.07.072.
Zhang G, Chai H, Tian M, Zhu S, Qu L, Zhang X. Zirconium-metalloporphyrin frameworks-luminol competitive electrochemiluminescence for ratiometric detection of polynucleotide kinase activity. Anal Chem. 2020;92(10):7354–62. https://doi.org/10.1021/acs.analchem.0c01262.
Song Z, Li Y, Teng H, Ding C, Xu G, Luo X. Designed zwitterionic peptide combined with sacrificial Fe-MOF for low fouling and highly sensitive electrochemical detection of T4 polynucleotide kinase. Sensors Actuators B Chem. 2020;305: 127329. https://doi.org/10.1016/j.snb.2019.127329.
Zhang Q, Li Z, Zhou Y, Li X, Li B, Yin H, Ai S. Electrochemical biosensors for polynucleotide kinase activity assay and inhibition screening based on phosphorylation reaction triggered λ exonuclease and exonuclease I cleavage. Sensors Actuators B Chem. 2016;225:151–7. https://doi.org/10.1016/j.snb.2015.11.033.
Hou T, Wang X, Liu X, Pan C, Li F. Sensitive electrochemical assay for T4 polynucleotide kinase activity based on dual-signaling amplification coupled with exonuclease reaction. Sensors Actuators B Chem. 2014;202:588–93. https://doi.org/10.1016/j.snb.2014.06.003.
Zhang Y, Fang X, Zhu Z, Lai Y, Xu C, Pang P, Wang H, Yang C, Barrow CJ, Yang W. A sensitive electrochemical assay for T4 polynucleotide kinase activity based on titanium dioxide nanotubes and a rolling circle amplification strategy. RSC Adv. 2018;8(67):38436–44. https://doi.org/10.1039/C8RA07745B.
Mao J, Chen X, Xu H, Xu X. DNAzyme-driven DNA walker biosensor for amplified electrochemical detection of T4 polynucleotide kinase activity and inhibition. J Electroanal Chem. 2020;874: 114470. https://doi.org/10.1016/j.jelechem.2020.114470.
Du J, Xu Q, Lu X, Zhang CY. A label-free bioluminescent sensor for real-time monitoring polynucleotide kinase activity. Anal Chem. 2014;86(16):8481–8. https://doi.org/10.1021/ac502240c.
Li P, Cao Y, Mao C, Jin B, Zhu J. TiO2/g-C3N4/CdS nanocomposite-based photoelectrochemical biosensor for ultrasensitive evaluation of T4 polynucleotide kinase activity. Anal Chem. 2019;91(2):1563–70. https://doi.org/10.1021/acs.analchem.8b04823.
Yan Z, Shen X, Zhou B, Pan R, Zhang B, Zhao C, Ren L, Ming J. Precise analysis of T4 polynucleotide kinase and inhibition by coupling personal glucose meter with split DNAzyme and ligation-triggered DNA walker. Sensors Actuators B Chem. 2021;326: 128831. https://doi.org/10.1016/j.snb.2020.128831.
Jiang H, Xu Y, Dai L, Liu X, Kong D. Ultrasensitive, label-free detection of T4 ligase and T4 polynucleotide kinase based on target-triggered hyper-branched rolling circle amplification. Sensors Actuators B Chem. 2018;260:70–7. https://doi.org/10.1016/j.snb.2017.12.203.
Wu X, He S, Zhao JX. Label-free fluorescence assay coupled exonuclease reaction and SYBR Green I for the detection of T4 polynucleotide kinase activity. Anal Methods. 2020;12(6):807–12. https://doi.org/10.1039/C9AY02283J.
Chen H, Wang Z, Chen X, Lou K, Sheng A, Chen T, Chen G, Zhang J. New method for detection of T4 polynucleotide kinase phosphatase activity through isothermal EXPonential amplification reaction. Analyst. 2019;144(6):1955–9. https://doi.org/10.1039/c8an02368a.
Li X, Xu X, Song J, Xue Q, Li C, Jiang W. Sensitive detection of T4 polynucleotide kinase activity based on multifunctional magnetic probes and polymerization nicking reactions mediated hyperbranched rolling circle amplification. Biosens Bioelectron. 2017;91:631–6. https://doi.org/10.1016/j.bios.2017.01.022.
Wang M, Kong D, Su D, Liu Y, Su X. Ratio fluorescence analysis of T4 polynucleotide kinase activity based on the formation of a graphene quantum dot-copper nanocluster nanohybrid. Nanoscale. 2019;11(29):13903–8. https://doi.org/10.1039/c9nr02901j.
Wang M, Chen J, Jiang S, Nie Y, Su X. Rapid synthesis of dual proteins co-functionalized gold nanoclusters for ratiometric fluorescence sensing of polynucleotide kinase activity. Sensors Actuators B Chem. 2021;329: 129200. https://doi.org/10.1016/j.snb.2020.129200.
Li J, Ma J, Zhang Y, Zhang Z, He G. A fluorometric method for determination of the activity of T4 polynucleotide kinase by using a DNA-templated silver nanocluster probe. Mikrochim Acta. 2019;186(1):48–54. https://doi.org/10.1007/s00604-018-3157-z.
Zhao H, Yan Y, Chen M, Hu T, Wu K, Liu H, Ma C. Exonuclease III-assisted signal amplification strategy for sensitive fluorescence detection of polynucleotide kinase based on poly(thymine)-templated copper nanoparticles. Analyst. 2019;144(22):6689–97. https://doi.org/10.1039/c9an01659g.
Zhu J, Chen L. Highly efficient incorporation of dATP in terminal transferase polymerization forming the ploy (A)n-DITO-1 fluorescent probe sensing terminal transferase and T4 polynucleotide kinase activity. Anal Chim Acta. 2022;1221: 340080. https://doi.org/10.1016/j.aca.2022.340080.
Huang Z, Zou J, Li X, He Q, Nie J. Research progress of fluorescent probe for G-quadruplex. Chin J Anal Chem 2021;49(8):1258–1269. https://doi.org/10.19756/j.issn.0253-3820.201797.
Zhou F, Wang G, Shi D, Sun Y, Sha L, Qiu Y, Zhang X. One-strand oligonucleotide probe for fluorescent label-free “turn-on” detection of T4 polynucleotide kinase activity and its inhibition. Analyst. 2015;140(16):5650–5. https://doi.org/10.1039/c5an00862j.
Mohanty J, Barooah N, Dhamodharan V, Harikrishna S, Pradeepkumar PI, Bhasikuttan AC. Thioflavin T as an efficient inducer and selective fluorescent sensor for the human telomeric G-quadruplex DNA. J Am Chem Soc. 2013;135(1):367–76. https://doi.org/10.1021/ja309588h.
Huang C, Shen G, Ding S, Kan A, Jiang D, Jiang W. Primer-template conversion-based cascade signal amplification strategy for sensitive and accurate detection of polynucleotide kinase activity. Anal Chim Acta. 2021;1187: 339139. https://doi.org/10.1016/j.aca.2021.339139.
Zhao H, Liu Q, Liu M, Jin Y, Li B. Label-free fluorescent assay of T4 polynucleotide kinase phosphatase activity based on G-quadruplex-thioflavin T complex. Talanta. 2017;165:653–8. https://doi.org/10.1016/j.talanta.2017.01.027.
Wang C, Li J. Fluorescence method for kanamycin detection based on the conversion of G-triplex and G-quadruplex. Anal Bioanal Chem. 2021;413(28):7073–80. https://doi.org/10.1007/s00216-021-03676-y.
Shi Z, Zhang X, Cheng R, Li B, Jin Y. A label-free cyclic assembly of G-quadruplex nanowires for cascade amplification detection of T4 polynucleotide kinase activity and inhibition. Analyst. 2015;140(17):6124–30. https://doi.org/10.1039/c5an00968e.
Cheng R, Tao M, Shi Z, Zhang X, Jin Y, Li B. Label-free and sensitive detection of T4 polynucleotide kinase activity via coupling DNA strand displacement reaction with enzymatic-aided amplification. Biosen Bioelectron. 2015;73:138–45. https://doi.org/10.1016/j.bios.2015.05.059.
Guo Y, Wang Q, Wang Z, Chen X, Xu L, Hu J, Pei R. Label-free detection of T4 DNA ligase and polynucleotide kinase activity based on toehold-mediated strand displacement and split G-quadruplex probes. Sensors Actuators B Chem. 2015;214:50–5. https://doi.org/10.1016/j.snb.2015.03.013.
Deng S, Zhou B, Li W, Li H, Zhang F, Ming J. Label-free fluorescence DNA walker for protein analysis based on terminal protection and dual enzyme assisted cleavage induced G-quadruplex/berberine conformation. Analyst. 2020;145(1):46–51. https://doi.org/10.1039/C9AN01853K.
Cheng Y, Cheng M, Hao J, Miao W, Zhou W, Jia G, Li C. Highly selective detection of K+ based on a dimerized G-quadruplex DNAzyme. Anal Chem. 2021;93(18):6907–12. https://doi.org/10.1021/acs.analchem.1c00872.
Ma G, Yu Z, Zhou W, Li Y, Fan L, Li X. Investigation of Na+ and K+ competitively binding with a G-quadruplex and discovery of a stable K+-Na+-quadruplex. J Phys Chem B. 2019;123(26):5405–11. https://doi.org/10.1021/acs.jpcb.9b02823.
Wang Z, Li M, Hsu SD, Chang T. Structural basis of sodium-potassium exchange of a human telomeric DNA quadruplex without topological conversion. Nucleic Acids Res. 2014;42(7):4723–33. https://doi.org/10.1093/nar/gku083.
Sun H, Xiang J, Gai W, Liu Y, Guan A, Yang Q, Li Q, Shang Q, Su H, Tang Y, Xu G. Quantification of the Na+/K+ ratio based on the different response of a newly identified G-quadruplex to Na+ and K+. Chem Commun. 2013;49(40):4510–2. https://doi.org/10.1039/c3cc39020a.
Jing S, Liu Q, Jin Y, Li B. Dimeric G-quadruplex: an effective nucleic acid scaffold for lighting up thioflavin T. Anal Chem. 2021;93(3):1333–41. https://doi.org/10.1021/acs.analchem.0c02637.
Song X, Ding Q, Pu Y, Zhang J, Sun R, Yin L, Wei W, Liu S. Application of the dimeric G-quadruplex and toehold-mediated strand displacement reaction for fluorescence biosensing of ochratoxin A. Biosens Bioelectron. 2021;192: 113537. https://doi.org/10.1016/j.bios.2021.113537.
Ma X, Lv M, Du F, Wu C, Lou B, Zeid AM, Xu G. Dimeric G-quadruplex: an efficient probe for ultrasensitive fluorescence detection of mustard compounds. Anal Chem. 2022;94(9):4112–8. https://doi.org/10.1021/acs.analchem.2c00124.
Jin T, Zhang J, Zhao Y, Huang X, Tan C, Sun S, Tan Y. Magnetic bead-gold nanoparticle hybrids probe based on optically countable gold nanoparticles with dark-field microscope for T4 polynucleotide kinase activity assay. Biosens Bioelectron. 2020;150:11936. https://doi.org/10.1016/j.bios.2019.11936.
Cui L, Li Y, Lu M, Tang B, Zhang C. An ultrasensitive electrochemical biosensor for polynucleotide kinase assay based on gold nanoparticle-mediated lambda exonuclease cleavage-induced signal amplification. Biosens Bioelectron. 2018;99:1–7. https://doi.org/10.1016/j.bios.2017.07.028.
Lin M, Wan H, Zhang J, Wang Q, Hu X, Xia F. Electrochemical DNA sensors based on MoS2-AuNPs for polynucleotide kinase activity and inhibition assay. ACS Appl Mater Interfaces. 2020;12(41):45814–21. https://doi.org/10.1021/acsami.0c13385.
Funding
This work was supported by the PhD Foundation of Weifang Medical University and the Public Domestic Visiting Program of Weifang Medical University (20217–13).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
This work has been approved by the Ethics Committee of Weifang Medical University and has been performed in accordance with the ethical standards.
Source of biological material
Human serum samples were obtained from the Affiliated Hospital of Weifang Medical University (Weifang, China). All serum-providing participants provided written informed consent for this study protocol.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, L., Kong, X., Wang, M. et al. A label-free T4 polynucleotide kinase fluorescence sensor based on split dimeric G-quadruplex and ligation-induced dimeric G-quadruplex/thioflavin T conformation. Anal Bioanal Chem 414, 7923–7933 (2022). https://doi.org/10.1007/s00216-022-04327-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04327-6