Abstract
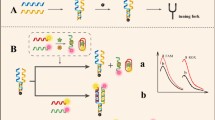
The simultaneous detection of multiple mycotoxins is of great significance for food safety and human health. Herein, a simple, convenient and accurate fluorescent aptasensor was designed based on the dual cross DNA nanostructure for the simultaneous detection of ochratoxin A (OTA) and aflatoxin B1 (AFB1), in which the stable dual cross DNA nanostructure provided an assay platform using the fluorescent dye-labeled aptamers as a sensing element. Owing to the higher affinity of aptamers for their target, the aptamer probes were released from the assay platform in the presence of OTA and AFB1, resulting in an enhanced fluorescence at 570 nm and 670 nm. This “signal-on” fluorescent aptasensor assay system can effectively avoid background signals and minimize false positive. Furthermore, the designed method can realize the simultaneous detection of OTA and AFB1 during the whole experiment. The limits of detection (LOD) were as low as 0.0058 ng/mL for OTA, ranging from 0.01 to 50 ng/mL and 0.046 ng/mL for AFB1, ranging from 0.05 to 100 ng/mL. The proposed fluorescent aptasensor exhibits excellent performance in practical application and provides a novel approach for the simultaneous detection of multiple mycotoxins by simply changing the aptamers.
Graphical abstract
A “signal-on” fluorescent aptasensor assay system based on the stable dual cross DNA nanostructure was successfully developed for simultaneous detection of OTA and AFB1 with lower detection limits in wider linear ranges.







Similar content being viewed by others
References
Zhang N, Liu BS, Cui XL, Li YT, Tang J, Wang HX, Zhang D, Li Z. Recent advances in aptasensors for mycotoxin detection: on the surface and in the colloid. Talanta. 2021;223(1):121729. https://doi.org/10.1016/j.talanta.2020.121729.
Jiang Q, Wu JD, Yao K, Yin YL, Max G, Yang CB, Francis L. Paper-based microfluidic device (DON-Chip) for rapid and low-cost deoxynivalenol quantification in food, feed, and feed ingredients. ACS Sens. 2019;4(11):3072–9. https://doi.org/10.1021/acssensors.9b01895.
Li Y, Liu X, Lin Z. Recent developments and applications of surface plasmon resonance biosensors for the detection of mycotoxins in foodstuffs. Food Chem. 2012;132(3):1549–54. https://doi.org/10.1016/j.foodchem.2011.10.109.
Wu H, Wang HY, Wu J, Han GQ, Liu YL, Zou P. A novel fluorescent aptasensor based on exonuclease-assisted triple recycling amplification for sensitive and label-free detection of aflatoxin B1. J Hazard Mater. 2021;415(5):125584. https://doi.org/10.1016/j.jhazmat.2021.125584.
Chauhan R, Singh J, Sachdev T, Basu T, Malhotra B. Recent advances in mycotoxins detection. Biosens Bioelectron. 2016;81(15):532–45. https://doi.org/10.1016/j.bios.2016.03.004.
Wang J, Mukhtar H, Ma L, Pang Q, Wang X. VHH antibodies: reagents for mycotoxin detection in food products. Sensors. 2018;18(2):485. https://doi.org/10.3390/s18020485.
He TT, Zhou T, Wan H, Han QB, Ma YQ, Tan T, Wan YQ. One-step deep eutectic solvent strategy for efficient analysis of aflatoxins in edible oils. J Sci Food Agric. 2020;100(13):4840–8. https://doi.org/10.1002/jsfa.10544.
Jia YM, Wu F, Liu PL, Zhou GH, Yu B, Lou XD, Xia F. A label-free fluorescent aptasensor for the detection of aflatoxin B1 in food samples using AIEgens and graphene oxide. Talanta. 2009;198(1):71–7. https://doi.org/10.1016/j.talanta.2019.01.078.
Sergeyev T, Yarynka D, Piletska E, Linnik R, Zaporozhets O, Brovko O, Piletsky S, El'skaya A. Development of a smartphone-based biomimetic sensor for aflatoxin B1 detection using molecularly imprinted polymer membranes. Talanta. 2019;201:204–10. https://doi.org/10.1016/j.talanta.2019.04.016.
Yang XS, Shi DM, Zhu SM, Wang BJ, Zhang XJ, Wang GF. Portable aptasensor of aflatoxin B1 in bread based on a personal glucose meter and DNA walking machine. ACS Sens. 2018;3(7):1368–75. https://doi.org/10.1021/acssensors.8b00304.
Seok YG, Byun JY, Shim WB, Kim MG. A structure-switchable aptasensor for aflatoxin B1 detection based on assembly of an aptamer/split DNAzyme. Anal Chim Acta. 2015;886(30):182–7. https://doi.org/10.1016/j.aca.2015.05.041.
Tang XQ, Li PW, Zhang Q, Zhang ZW, Zhang W, Jiang J. Time-resolved fluorescence immunochromatographic assay developed using two idiotypic nanobodies for rapid, quantitative, and simultaneous detection of aflatoxin and Zearalenone in maize and its products. Anal Chem. 2017;89(21):11520–8. https://doi.org/10.1021/acs.analchem.7b02794.
Suo ZG, Liu XW, Hou XL, Liu Y, Lu JT, Xing FF, Chen YY, Feng LY. Ratiometric assays for acetylcholinesterase activity and organo-phosphorous pesticide based on superior carbon quantum dots and BLGF-protected gold nanoclusters FRET process. ChemistrySelect. 2020;5(29):9254–60. https://doi.org/10.1002/slct.202002042.
Rasooly R, Do PM, Hernlem BJ. Low cost quantitative digital imaging as an alternative to qualitative in vivo bioassays for analysis of active aflatoxin B1. Biosens Bioelectron. 2016;80:405–10. https://doi.org/10.1016/j.bios.2016.01.087.
Zhou JH, Rossi J. Aptamers as targeted therapeutics: current potential and challenges. Nat Rev Drug Discov. 2017;16(3):2889–93. https://doi.org/10.1038/nrd.2016.199.
Zhao BJ, Wu P, Zhang H, Cai CX. Designing activatable aptamer probes for simultaneous detection of multiple tumor-related proteins in living cancer cells. Biosens Bioelectron. 2015;68(15):763–70. https://doi.org/10.1016/j.bios.2015.02.004.
Suo ZG, Chen JQ, Hou XL, Hu ZH, Xing FF, Feng LY. Growing prospects of DNA nanomaterials in novel biomedical applications. RSC Adv. 2019;9(29):16479–91. https://doi.org/10.1039/c9ra01261c.
Nameghi MA, Danesh NM, Ramezani M, Hassani FV, Abnous K, Taghdisi SM. A fluorescent aptasensor based on a DNA pyramid nanostructure for ultrasensitive detection of Ochratoxin a. Anal Bioanal Chem. 2016;408(21):5811–8. https://doi.org/10.1007/s00216-016-9693-7.
Zhong L, Cai SX, Huang YQ, Yin LT, Yang YL, Lu CH, Yang HH. A DNA octahedron-based fluorescence nanoprobe for dual tumorrelated mRNAs detection and imaging. Anal Chem. 2018;90(20):12059–66. https://doi.org/10.1021/acs.analchem.8b02847.
Wei M, He X, Xie YL. A novel signal-on fluorescent aptasensor for Ochratoxin a detection based on RecJf exonuclease-induced signal amplification. J Chin Chem Soc. 2020;67(7):1247–53. https://doi.org/10.1002/jccs.201900423.
Wang Y, Hu XF, Pei YF, Sun YN, Wang FY, Song CM, Yin MQ, Deng RG, Li ZX, Zhang GPY. Selection of phage-displayed minotopes of ochratoxin a and its detection in cereal by ELISA. Anal Methods. 2015;7(5):1849–54. https://doi.org/10.1039/c4ay02290d.
AntonellaVatinno A, Palmisano R, Zambonin F, Carlo G. Determination of Ochratoxin a in wine at sub ng/mL levels by solid-phase microextraction coupled to liquid chromatography with fluorescence detection. J Chromatogr A. 2006;1115(1–2):196–201. https://doi.org/10.1016/j.chroma.2006.02.092.
Gke G, Aissa SB, Nemčekov K, Catanante G, Raouafi N, Marty JL. Aptamer-modified pencil graphite electrodes for the impedimetric determination of Ochratoxin a. Food Control. 2020;115:107271. https://doi.org/10.1016/j.foodcont.2020.107271.
Bulbul G, Hayat A, Andreescu S. A generic amplification strategy for electrochemical aptasensors using a non-enzymatic Nanoceria tag. Nanoscale. 2015;7(31):13230–8. https://doi.org/10.1039/c5nr02628h.
Lin CY, Zheng HX, Sun M, Guo YJ, Luo F, Guo LH, Qiu B, Lin ZY, Chen GN. Highly sensitive colorimetric aptasensor for ochratoxin a detection based on enzyme-encapsulated liposome. Anal Chim Acta. 2018;1002(9):90–6. https://doi.org/10.1016/j.aca.2017.11.061.
Yin XT, Wang S, Liu XY, He CM, Tang YL, Li QM, Liu JH, Su HJ, Tan TW, Dong YY. Aptamer-based colorimetric biosensing of Ochratoxin a in fortified white grape wine sample using unmodified gold nanoparticles. Anal Sci. 2017;33(6):659–64. https://doi.org/10.2116/analsci.33.659.
Hao L, Wang W, Shen X, Wang SL, Li Q, An FL, Wu SJ. A fluorescent DNA hydrogel aptasensor based on the self-assembly of rolling circle amplification products for sensitive detection of Ochratoxin a. J Agric Food Chem. 2020;68(1):369–75. https://doi.org/10.1021/acs.jafc.9b06021.
Wu KF, Ma CB, Zhao H, Chen MJ, Deng ZY. Sensitive aptamer-based fluorescene assay for Ochratoxin a based on RNase H signal amplification. Food Chem. 2019;277(30):273–8. https://doi.org/10.1016/j.foodchem.2018.10.130.
Zhao FC, Tian Y, Shen Q, Liu RX, Shi RR, Wang HM, Yang ZY. A novel nanobody and mimotope based immunoassay for rapid analysis of aflatoxin B1. Talanta. 2019;195:55–61. https://doi.org/10.1016/j.talanta.2018.11.013.
Nonaka Y, Saito K, Hanioka N, Narimatsu S, Kataoka H. Determination of aflatoxins in food samples by automated on-line in-tubesolid-phase microextraction coupled with liquid chromatography–massspectrometry. J Chromatogr A. 2009;1216(20):4416–22. https://doi.org/10.1016/j.chroma.2009.03.035.
Lai WQ, Zeng Q, Tang J, Zhang MS, Tang DP (2018) A conventional chemical reaction for use in an unconventional assay: a colorimetric immunoassay for aflatoxin B1 by using enzyme-responsive just-in-time generation of a MnO2 based nanocatalyst. Microchim Acta 185(2):92. https://doi.org/10.1007/s00604-017-2651-z.
Goud KY, Hayat A, Catanante G, Satyanarayana M, Gobi KV, Marty JL. An electrochemical aptasensor based on functionalized graphene oxide assisted electrocatalytic signal amplification of methylene blue for aflatoxin B1 detection. Electrochim Acta. 2017;244(1):96–103. https://doi.org/10.1016/j.electacta.2017.05.089.
Li X, Yang L, Men C, Xie YF, Liu JJ, Zou HY, Li YF, Zhan L, Huang CZ. Photothermal soft nanoballs developed by loading plasmonic Cu2-XSe nanocrystals into liposomes for photothermal immunoassay of aflatoxin B1. Anal Chem. 2019;91(7):4444–50. https://doi.org/10.1021/acs.analchem.8b05031.
Tan HX, Ma L, Guo T, Zhou HY, Chen L, Zhang YH, Dai HJ, Yu Y. A novel fluorescence aptasensor based on mesoporous silica nanoparticles for selective and sensitive detection of aflatoxin B1. Anal Chim Acta. 2019;1068(30):87–95. https://doi.org/10.1016/j.aca.2019.04.014.
Funding
This study was funded by the Key Scientific and Technological Project of Henan Province (212102310001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 27 kb)
Rights and permissions
About this article
Cite this article
Suo, Z., Liang, X., Jin, H. et al. A signal-enhancement fluorescent aptasensor based on the stable dual cross DNA nanostructure for simultaneous detection of OTA and AFB1. Anal Bioanal Chem 413, 7587–7595 (2021). https://doi.org/10.1007/s00216-021-03723-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03723-8