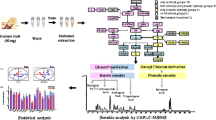
Abstract
The interest in metabolomic studies has rapidly increased over the past few years. Changes of endogenous compounds are typically detected in plasma or urine. However, the use of hair allows for long-term monitoring of metabolomic changes and has recently started being applied to metabolomic studies. Within the proposed study, we aimed for a systematical investigation of different pre-analytical parameters on detected metabolites from different chemical classes in hair. For this purpose, three different parameters were varied: (1) multi-step decontamination (dichloromethane (DCM), acetone, H2O, acetone; H2O, acetone, DCM, acetone; and H2O, methanol/acetone), (2) homogenization (pulverization vs. cutting into snippets), and (3) extraction (acetonitrile (ACN)/buffer pH 4 vs. ACN/H2O vs. ACN/buffer pH 8.5). To include as many metabolites as possible, samples were analyzed by high-resolution time of flight mass spectrometry coupled to liquid chromatography (HPLC-HRMS) and additionally by gas chromatography high-resolution mass spectrometry (GC-HRMS) followed by untargeted-like data processing, respectively. The application of different decontamination procedures yielded similar results, although pointing to a trend towards increased washing-out effects if protic solvents were used as a first washing step. Pulverization of hair samples was favorable in terms of detected and tentatively identified metabolites. Evaluation of extraction solvents showed differences in extraction yield for the majority of investigated metabolites, yet, a prediction of metabolite extraction according to their pKa values was not possible. Overall, successive decontamination with DCM, acetone, H2O, and acetone; homogenization by pulverization; and extraction with ACN/H2O produced reliable results.

Graphical abstract





Similar content being viewed by others
References
Patti GJ, Yanes O, Innovation SG. Metabolomics: the apogee of the omics trilogy. Nat Rev Mol Cell Biol. 2012;13(4):263–9.
Dinis-Oliveira RJ. Metabolomics of drugs of abuse: a more realistic view of the toxicological complexity. Bioanalysis. 2014;6(23):3155–9.
Dettmer K, Aronov PA, Hammock BD. Mass spectrometry-based metabolomics. Mass Spectrom Rev. 2007;26(1):51–78. https://doi.org/10.1002/mas.20108.
Nicholson JK, Lindon JC, Holmes E. ‘Metabonomics’: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999;29(11):1181–9.
Monteiro MS, Carvalho M, Bastos ML, Guedes de Pinho P. Metabolomics analysis for biomarker discovery: advances and challenges. Curr Med Chem. 2013;20(2):257–71.
Boxler MI, Liechti ME, Schmid Y, Kraemer T, Steuer AE. First time view on human metabolome changes after a single intake of 3,4-methylenedioxymethamphetamine in healthy placebo-controlled subjects. J Proteome Res. 2017;16(9):3310–20.
Pragst F, Balikova MA. State of the art in hair analysis for detection of drug and alcohol abuse. Clin Chim Acta. 2006;370(1–2):17–49.
Binz TM, Rietschel L, Streit F, Hofmann M, Gehrke J, Herdener M, et al. Endogenous cortisol in keratinized matrices: systematic determination of baseline cortisol levels in hair and the influence of sex, age and hair color. Forensic Sci Int. 2018;284:33–8.
Binz TM, Braun U, Baumgartner MR, Kraemer T. Development of an LC-MS/MS method for the determination of endogenous cortisol in hair using (13)C3-labeled cortisol as surrogate analyte. J Chromatogr B Anal Technol Biomed Life Sci. 2016;1033-1034:65–72.
Greff MJE, Levine JM, Abuzgaia AM, Elzagallaai AA, Rieder MJ, van Uum SHM. Hair cortisol analysis: an update on methodological considerations and clinical applications. Clin Biochem. 2019;63:1–9.
Rashaid AH, Harrington Pde B, Jackson GP. Profiling amino acids of Jordanian scalp hair as a tool for diabetes mellitus diagnosis: a pilot study. Anal Chem. 2015;87(14):7078–84.
Masukawa Y, Narita H, Imokawa G. Characterization of the lipid composition at the proximal root regions of human hair. J Cosmet Sci. 2005;56(1):1–16.
Delplancke TDJ, de Seymour JV, Tong C, Sulek K, Xia Y, Zhang H, et al. Analysis of sequential hair segments reflects changes in the metabolome across the trimesters of pregnancy. Sci Rep. 2018;8(1):36.
Jones B, Han TL, Delplancke T, McKenzie EJ, de Seymour JV, Chua MC, et al. Association between maternal exposure to phthalates and lower language ability in offspring derived from hair metabolome analysis. Sci Rep. 2018;8(1):6745.
Sulek K, Han TL, Villas-Boas SG, Wishart DS, Soh SE, Kwek K, et al. Hair metabolomics: identification of fetal compromise provides proof of concept for biomarker discovery. Theranostics. 2014;4(9):953–9.
He X, de Seymour JV, Sulek K, Qi H, Zhang H, Han TL, et al. Maternal hair metabolome analysis identifies a potential marker of lipid peroxidation in gestational diabetes mellitus. Acta Diabetol. 2016;53(1):119–22.
Sumner LW, Amberg A, Barrett D, Beale MH, Beger R, Daykin CA, et al. Proposed minimum reporting standards for chemical analysis chemical analysis working group (CAWG) metabolomics standards initiative (MSI). Metabolomics. 2007;3(3):211–21.
Deda O, Chatziioannou AC, Fasoula S, Palachanis D, Raikos N, Theodoridis GA, et al. Sample preparation optimization in fecal metabolic profiling. J Chromatogr B Anal Technol Biomed Life Sci. 2017;1047:115–23.
Raterink RJ, Lindenburg PW, Vreeken RJ, Ramautar R, Hankemeier T. Recent developments in sample-pretreatment techniques for mass spectrometry-based metabolomics. TrAC Trends Anal Chem. 2014;61:157–67.
Yin P, Lehmann R, Xu G. Effects of pre-analytical processes on blood samples used in metabolomics studies. Anal Bioanal Chem. 2015;407(17):4879–92.
Vogliardi S, Tucci M, Stocchero G, Ferrara SD, Favretto D. Sample preparation methods for determination of drugs of abuse in hair samples: a review. Anal Chim Acta. 2015;857:1–27.
Society of Hair Testing. Recommendations for hair testing in forensic cases. Forensic Sci Int. 2004;145(2–3):83–4.
Kintz P, Cirimele V, Jamey C, Ludes B. Testing for GHB in hair by GC/MS/MS after a single exposure. Application to document sexual assault. J Forensic Sci. 2003;48(1):195–200.
Choi B, Kim SP, Hwang S, Hwang J, Yang CH, Lee S. Metabolic characterization in urine and hair from a rat model of methamphetamine self- administration using LC- QTOF-MS-based metabolomics. Metabolomics. 2017. https://doi.org/10.1007/s11306-017-1257-0.
Xie P, Wang TJ, Yin G, Yan Y, Xiao LH, Li Q, et al. Metabonomic study of biochemical changes in human hair of heroin abusers by liquid chromatography coupled with ion trap-time of flight mass spectrometry. J Mol Neurosci. 2016;58(1):93–101.
Madry MM, Kraemer T, Baumgartner MR. Systematic assessment of different solvents for the extraction of drugs of abuse and pharmaceuticals from an authentic hair pool. Forensic Sci Int. 2018;282:137–43.
Dunn WB, Broadhurst D, Begley P, Zelena E, Francis-McIntyre S, Anderson N, et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat Protoc. 2011;6(7):1060–83.
Steuer AE, Arnold K, Kamber D, Kraemer T. Suitability evaluation of new endogenous biomarkers for the identification of nitrite-based urine adulteration in mass spectrometry methods. Drug Test Anal. 2018. https://doi.org/10.1002/dta.2481.
Boxler MI, Schneider TD, Kraemer T, Steuer AE. Analytical considerations for (un)-targeted metabolomic studies with special focus on forensic applications. Drug Test Anal. 2018. https://doi.org/10.1002/dta.2540.
Brockbals L, Habicht M, Hajdas I, Galassi FM, Ruhli FJ, Kraemer T. Untargeted metabolomics-like screening approach for chemical characterization and differentiation of canopic jar and mummy samples from Ancient Egypt using GC-high resolution MS. Analyst. 2018;143(18):4503–12.
Linstrom PJ, Mallard WG. NIST Chemistry WebBook. NIST Standard Reference Database Number 69, 2001.
Guijas C, Montenegro-Burke JR, Domingo-Almenara X, Palermo A, Warth B, Hermann G, et al. METLIN: a technology platform for identifying knowns and unknowns. Anal Chem. 2018;90(5):3156–64.
Kind T, Liu KH, Lee DY, DeFelice B, Meissen JK, Fiehn O. LipidBlast in silico tandem mass spectrometry database for lipid identification. Nat Methods. 2013;10(8):755–8.
Wishart DS, Tzur D, Knox C, Eisner R, Guo AC, Young N, et al. HMDB: the human metabolome database. Nucleic Acids Res. 2007. https://doi.org/10.1093/nar/gkl923.
Zhu X, Chen Y, Subramanian R. Comparison of information-dependent acquisition, SWATH, and MS(All) techniques in metabolite identification study employing ultrahigh-performance liquid chromatography-quadrupole time-of-flight mass spectrometry. Anal Chem. 2014;86(2):1202–9.
Noppe G, de Rijke YB, Dorst K, van den Akker EL, van Rossum EF. LC-MS/MS-based method for long-term steroid profiling in human scalp hair. Clin Endocrinol. 2015;83(2):162–6.
Cuykx M, Rodrigues RM, Laukens K, Vanhaecke T, Covaci A. In vitro assessment of hepatotoxicity by metabolomics: a review. Arch Toxicol. 2018;92(10):3007–29.
Cooper GA, Kronstrand R, Kintz P, Society of Hair Testing. Society of Hair Testing guidelines for drug testing in hair. Forensic Sci Int. 2012;218(1–3):20–4.
De AK, Chowdhury PP, Chattapadhyay S. Simultaneous quantification of dexpanthenol and resorcinol from hair care formulation using liquid chromatography: method development and validation. Scientifica (Cairo). 2016;2016:1537952.
Cosmetic Ingredient Review Expert P. Final report of the safety assessment of niacinamide and niacin. Int J Toxicol. 2005;24(Suppl 5):1–31.
Fiume MM, Heldreth B, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, et al. Safety assessment of triethanolamine and triethanolamine-containing ingredients as used in cosmetics. Int J Toxicol. 2013;32(3 Suppl):59S–83S.
Delgado-Povedano MM, Calderon-Santiago M, Priego-Capote F, Luque de Castro MD. Development of a method for enhancing metabolomics coverage of human sweat by gas chromatography-mass spectrometry in high resolution mode. Anal Chim Acta. 2016;905:115–25.
Camera E, Ludovici M, Galante M, Sinagra JL, Picardo M. Comprehensive analysis of the major lipid classes in sebum by rapid resolution high-performance liquid chromatography and electrospray mass spectrometry. J Lipid Res. 2010;51(11):3377–88.
Eser HP, Potsch L, Skopp G, Moeller MR. Influence of sample preparation on analytical results: drug analysis [GC/MS] on hair snippets versus hair powder using various extraction methods. Forensic Sci Int. 1997;84(1–3):271–9.
Salomone A, Baumgartner MR, Lombardo T, Alladio E, Di Corcia D, Vincenti M. Effects of various sample pretreatment procedures on ethyl glucuronide quantification in hair samples: comparison of positivity rates and appraisal of cut-off values. Forensic Sci Int. 2016;267:60–5.
Monch B, Becker R, Nehls I. Quantification of ethyl glucuronide in hair: effect of milling on extraction efficiency. Alcohol Alcohol. 2013;48(5):558–63.
Aqai P, Stolker AA, Lasaroms JJ. Effect of sample pre-treatment on the determination of steroid esters in hair of bovine calves. J Chromatogr A. 2009;1216(46):8233–9.
Becker R, Lo I, Sporkert F, Baumgartner M. The determination of ethyl glucuronide in hair: experiences from nine consecutive interlaboratory comparison rounds. Forensic Sci Int. 2018;288:67–71.
Chata C, E MH, Grova N, Appenzeller BM. Influence of pesticide physicochemical properties on the association between plasma and hair concentration. Anal Bioanal Chem. 2016;408(13):3601–12.
Acknowledgements
The authors would like to express their gratitude to Emma Louise Kessler, MD, for her generous legacy; she donated to the Institute of Forensic Medicine at the University of Zurich, Switzerland, for research purposes.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Hair samples were collected from two healthy volunteers who provided written informed consent. According to Swissethics (Humanforschungsgesetz), no further ethical approval from the cantonal ethic commission is necessary if the research is not aiming to investigate diseases or functions of the human body as is the case in the current study.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 231 kb)
Rights and permissions
About this article
Cite this article
Eisenbeiss, L., Steuer, A.E., Binz, T.M. et al. (Un)targeted hair metabolomics: first considerations and systematic evaluation on the impact of sample preparation. Anal Bioanal Chem 411, 3963–3977 (2019). https://doi.org/10.1007/s00216-019-01873-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-01873-4