Abstract
Rationale
The long-term effectiveness of olanzapine and aripiprazole in real clinical conditions at flexible doses in patients after hospital discharge has not been evaluated yet.
Objectives
This study was a multicenter retrospective cohort study. Patients with schizophrenia (n = 398) were prescribed olanzapine (n = 303) or aripiprazole (n = 95) at hospital discharge. The continuation of olanzapine or aripiprazole at 26, 52, or 104 weeks after the hospital discharge were compared using a Cox proportional hazards model and adjusted for possible confounders.
Results
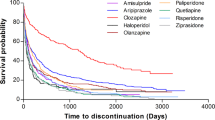
The Kaplan–Meier survival curves revealed that the continuation of olanzapine at 26 (P = 0.001) and 52 weeks (P = 0.018) was significantly higher than that of aripiprazole but not at 104 weeks. Olanzapine was better than aripiprazole in efficacy at 26 (hazard ratio: 0.321, 95% confidence interval: 0.159–0.645, P = 0.001), 52 (hazard ratio: 0.405, 95% confidence interval: 0.209–0.786, P = 0.008), and 104 weeks (hazard ratio: 0.438, 95% confidence interval: 0.246–0.780, P = 0.005). Aripiprazole was better than olanzapine in tolerability at 104 weeks (hazard ratio: 4.574, 95% confidence interval: 1.415–14.787, P = 0.011). Rates after two years continuation of olanzapine and aripiprazole were not significantly different in patients with less than five years' duration of illness, but olanzapine was more commonly maintained for more than two years in those patients who had been ill for over five years' due to its greater efficacy.
Conclusion
Olanzapine treatment showed better continuation rates at 26 and 52 after hospital discharge than aripiprazole, whereas maintenance with the two antipsychotics did not differ significantly at 104 weeks, due reduced tolerability of long-term olanzapine treatment.

Similar content being viewed by others
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Álvarez-Jiménez M, González-Blanch C, Crespo-Facorro B et al (2008) Antipsychotic-induced weight gain in chronic and first-episode psychotic disorders: a systematic critical reappraisal. CNS Drugs 22:547–562. https://doi.org/10.2165/00023210-200822070-00002
Bak M, Fransen A, Janssen J et al (2014) Almost all antipsychotics result in weight gain: a meta-analysis. PLoS One 9:e94112. https://doi.org/10.1371/JOURNAL.PONE.0094112
Bećarević N, Softić R, Osmanović E (2022) Does the duration of the illness affect the severity of negative symptoms of schizophrenia? Mater Sociomed 34:25–27. https://doi.org/10.5455/MSM.2022.33.25-27
Bobes J, Garcia-Portilla MP, Bascaran MT, Saiz PA BM (2007) Quality of life in schizophrenic patients. Dialogues Clin Neurosci 9:215–226. https://doi.org/10.31887/DCNS.2007.9.2/JBOBES
Buoli M, Caldiroli A, Panza G, Altamura AC (2012) Prominent clinical dimension, duration of illness and treatment response in schizophrenia: a naturalistic study. Psychiatry Investig 9:354–360. https://doi.org/10.4306/PI.2012.9.4.354
Carnovale C, Lucenteforte E, Battini V et al (2021) Association between the glyco-metabolic adverse effects of antipsychotic drugs and their chemical and pharmacological profile: a network meta-analysis and regression. Psychol Med 24:1–13. https://doi.org/10.1017/S0033291721000180
Ceraso A, Lin JJ, Schneider-Thoma J et al (2020) Maintenance treatment with antipsychotic drugs for schizophrenia. Cochrane database Syst Rev 8:CD008016. https://doi.org/10.1002/14651858.CD008016.pub3
Cerovecki A, Musil R, Klimke A et al (2013) Withdrawal symptoms and rebound syndromes associated with switching and discontinuing atypical antipsychotics: theoretical background and practical recommendations. CNS Drugs 27:545–572. https://doi.org/10.1007/s40263-013-0079-5
Chrzanowski WK, Marcus RN, Torbeyns A et al (2006) Effectiveness of long-term aripiprazole therapy in patients with acutely relapsing or chronic, stable schizophrenia: a 52-week, open-label comparison with olanzapine. Psychopharmacology (Berl) 189:259–266. https://doi.org/10.1007/s00213-006-0564-3
Curson DA, Barnes TR, Bamber RW et al (1985) Long-term depot maintenance of chronic schizophrenic out-patients: the seven year follow-up of the Medical Research Council fluphenazine/placebo trial. III. Relapse postponement or relapse prevention? The implications for long-term outcome. Br J Psychiatry 146:474–480. https://doi.org/10.1192/bjp.146.5.474
Dayabandara M, Hanwella R, Ratnatunga S et al (2017) Antipsychotic-associated weight gain: management strategies and impact on treatment adherence. Neuropsychiatr Dis Treat 13:2231–2241. https://doi.org/10.2147/NDT.S113099
Emsley R, Chiliza B, Asmal L (2013) The evidence for illness progression after relapse in schizophrenia. Schizophr Res 148:117–121. https://doi.org/10.1016/j.schres.2013.05.016
Emsley R, Chiliza B, Asmal L, Harvey BH (2013) The nature of relapse in schizophrenia. BMC Psychiatry 13:50. https://doi.org/10.1186/1471-244X-13-50
Emsley R, Oosthuizen P, Koen L et al (2013) Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol 33:80–83. https://doi.org/10.1097/JCP.0b013e31827bfcc1
Faulkner G, Cohn T, Remington G (2007) Interventions to reduce weight gain in schizophrenia. Cochrane Database Syst Rev 1:CD005148. https://doi.org/10.1002/14651858.CD005148.pub2
Furukawa TA, Levine SZ, Tanaka S et al (2015) Initial severity of schizophrenia and efficacy of antipsychotics: participant-level meta-analysis of 6 placebo-controlled studies. JAMA psychiatry 72:14–21. https://doi.org/10.1001/jamapsychiatry.2014.2127
Gardner DM, Murphy AL, O’Donnell H et al (2010) International consensus study of antipsychotic dosing. Am J Psychiatry 167:686–693. https://doi.org/10.1176/appi.ajp.2009.09060802
Huhn M, Nikolakopoulou A, Schneider-Thoma J et al (2019) Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet (London, England) 394:939–951. https://doi.org/10.1016/S0140-6736(19)31135-3
Jain S, Bhargava M, Gautam S (2006) Weight gain with olanzapine: Drug, gender or age? Indian J Psychiatry 48:39–42. https://doi.org/10.4103/0019-5545.31617
Kahn RS, Fleischhacker WW, Boter H et al (2008) Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet (London, England) 371:1085–1097. https://doi.org/10.1016/S0140-6736(08)60486-9
Kane JM, Osuntokun O, Kryzhanovskaya LA et al (2009) A 28-week, randomized, double-blind study of olanzapine versus aripiprazole in the treatment of schizophrenia. J Clin Psychiatry 70:572–581. https://doi.org/10.4088/jcp.08m04421
Kane JM, Leucht S, Carpenter D, Docherty JP (2003) The expert consensus guideline series. Optimizing pharmacologic treatment of psychotic disorders. Introduction: methods, commentary, and summary. J Clin Psychiatry 64:5–19. Retrieved from https://www.psychiatrist.com/jcp/
Kasper S (1999) First-episode schizophrenia: the importance of early intervention and subjective tolerability. J Clin Psychiatry 60:5–9. Retrieved from https://www.psychiatrist.com/jcp/
Kinon BJ, Volavka J, Stauffer V et al (2008) Standard and higher dose of olanzapine in patients with schizophrenia or schizoaffective disorder: a randomized, double-blind, fixed-dose study. J Clin Psychopharmacol 28:392–400. https://doi.org/10.1097/jcp.0b013e31817e63a5
Leucht S, Crippa A, Siafis S et al (2020) Dose-Response Meta-Analysis of Antipsychotic Drugs for Acute Schizophrenia. Am J Psychiatry 177:342–353. https://doi.org/10.1176/appi.ajp.2019.19010034
Lieberman J, Jody D, Geisler S et al (1993) Time course and biologic correlates of treatment response in first-episode schizophrenia. Arch Gen Psychiatry 50:369–376. https://doi.org/10.1001/archpsyc.1993.01820170047006
Lieberman JA, Stroup TS, McEvoy JP et al (2005) Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 353:1209–1223. https://doi.org/10.1056/NEJMoa051688
McQuade RD, Stock E, Marcus R et al. (2004) A comparison of weight change during treatment with olanzapine or aripiprazole: results from a randomized, double-blind study. J Clin Psychiatry 65:47–56. Retrieved from https://www.psychiatrist.com/jcp/
Meltzer HY, Bobo WV, Roy A et al (2008) A randomized, double-blind comparison of clozapine and high-dose olanzapine in treatment-resistant patients with schizophrenia. J Clin Psychiatry 69:274–285. https://doi.org/10.4088/jcp.v69n0214
Obayashi Y, Mitsui S, Sakamoto S et al (2020) Switching strategies for antipsychotic monotherapy in schizophrenia: a multi-center cohort study of aripiprazole. Psychopharmacology (Berl) 237:167–175. https://doi.org/10.1007/s00213-019-05352-7
Pillinger T, McCutcheon RA, Vano L et al (2020) Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. The Lancet Psychiatry 7:64–77. https://doi.org/10.1016/S2215-0366(19)30416-X
Robinson D, Woerner MG, Alvir JMJ et al (1999) Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry 56:241–247. https://doi.org/10.1001/archpsyc.56.3.241
Schneider-Thoma J, Chalkou K, Dörries C et al (2022) Comparative efficacy and tolerability of 32 oral and long-acting injectable antipsychotics for the maintenance treatment of adults with schizophrenia: a systematic review and network meta-analysis. Lancet (London, England) 399:824–836. https://doi.org/10.1016/S0140-6736(21)01997-8
Shimomura Y, Kikuchi Y, Suzuki T et al (2020) Antipsychotic treatment in the maintenance phase of schizophrenia: An updated systematic review of the guidelines and algorithms. Schizophr Res 215:8–16. https://doi.org/10.1016/j.schres.2019.09.013
Stern RG, Kahn RSDM (1993) Predictors of response to neuroleptic treatment in schizophrenia. Psychiatr Clin North Am 16:313–338. https://doi.org/10.1016/S0193-953X(18)30176-X
Teasdale SB, Ward PB, Rosenbaum S et al (2017) Solving a weighty problem: systematic review and meta-analysis of nutrition interventions in severe mental illness. Br J Psychiatry 210:110–118. https://doi.org/10.1192/bjp.bp.115.177139
Thompson A, Winsper C, Marwaha S et al (2018) Maintenance antipsychotic treatment versus discontinuation strategies following remission from first episode psychosis: systematic review. BJPsych open 4:215–225. https://doi.org/10.1192/bjo.2018.17
Üçok A, Kara İA (2020) Relapse rates following antipsychotic discontinuation in the maintenance phase after first-episode of schizophrenia: Results of a long-term follow-up study. Schizophr Res 225:31–38. https://doi.org/10.1016/j.schres.2019.10.015
Zhu Y, Li C, Huhn M et al (2017) How well do patients with a first episode of schizophrenia respond to antipsychotics: A systematic review and meta-analysis. Eur Neuropsychopharmacol 27:835–844. https://doi.org/10.1016/j.euroneuro.2017.06.011
Acknowledgements
The authors would like to thank the Zikei Institute of Psychiatry (Okayama, Japan).
Funding
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Author information
Authors and Affiliations
Contributions
T. Hosokawa, Y. Yoshimura, K. Washida, Y. Yada, S. Sakamoto, Y. Okahisa, S. Takao, A. Nomura, Y. Kishi, T. Harada, M. Takaki, T. Takeda, and N. Yamada participated in the design of the study, supervised the project, and contributed intellectually to the interpretation of the data. T. Hosokawa, Y. Yoshimura, K. Washida, Y. Yada, A. Nomura, Y. Kishi, and T. Harada investigated patient clinical records. C. Miyaji and S. Takao performed the statistical analyses. All authors contributed to and have approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
N.Y. has received unrestricted research funding from Daiichi Sankyo, Eisai, Pfizer, Otsuka, Astellas, and Merck Sharp & Dohme, which was deposited into research accounts at Okayama University. N.Y. has received honoraria for his participation as a speaker at educational events from UCB Japan, Tsumura, Pfizer, Dainippon-Sumitomo, Daiichi-Sankyo, Merck Sharp & Dohme, Pfizer, Eisai, Meiji-Seika, and Mochida.
M.T. has received honoraria for his participation as a speaker at education events sponsored by Daiichi Sankyo, Takeda, Tsumura, Otsuka, and Dainippon Sumitomo.
Y. Yada has received honoraria for speaking at educational events sponsored by Novartis, Otsuka, and Dainippon Sumitomo.
S.S. has received unrestricted research funding from Eli Lilly, which was deposited into research accounts at Okayama University Hospital. S.S. has received honoraria for his participation as a speaker at an educational event sponsored by Otsuka and Meiji-Seika.
Y.K. has received honoraria for his participation as a speaker at educational events sponsored by Novartis and Dainippon Sumitomo.
T. Hosokawa, C.M., Y. Yoshimura, Y.O., K. Washida, S.T., A.N., T. Harada, and T.T. report no additional financial or other relationship relevant to this article.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hosokawa, T., Miyaji, C., Yoshimura, Y. et al. Comparison between olanzapine and aripiprazole treatment for 104 weeks after hospital discharge in schizophrenia spectrum disorders: a multicenter retrospective cohort study in a real-world setting. Psychopharmacology 240, 1911–1920 (2023). https://doi.org/10.1007/s00213-023-06407-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-023-06407-6