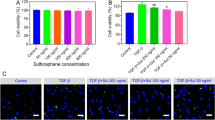
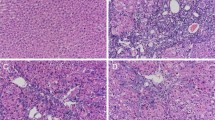
Abstract
Fibrotic kidney injury from hepatocarcinogenesis seriously impacts treatment effect. Astragaloside IV (AS-IV), an extract of Astragalus membranaceus, has several pharmacological activities, which are useful in the treatment of edema and fibrosis. Nrf2/HO-1 is a key antioxidant stress pathway and help treatment of kidney injury. Smad3 phosphorylation is implicated in hepatocarcinogenesis. Our previous study clarified that Smad3 is differentially regulated by different phosphorylated forms of Smad3 on hepatocarcinogenesis. Therefore, we investigated the contribution of AS-IV on the therapy of kidney fibrosis from hepatocarcinogenesis. And the focus was on whether the phosphorylation of Smad3 and the regulation of Nrf2/HO-1 pathway were involved during AS-IV therapy and whether there is an effect of Nrf2 knockout on the phosphorylation of Smad3. We performed TGF-β1 stimulation on HK-2 cells and intervened with AS-IV. Furtherly, we investigated renal injury of AS-IV on Nrf2 knockout mice during hepatocarcinogenesis and its mechanism of action. On the one hand, in vitro results showed that AS-IV reduced the ROS and α-SMA expression of HK-2 by promoting the expression pSmad3C/p21 of and Nrf2/HO-1 and suppressed the expression of pSmad3L/PAI-1. On the other hand, the in vivo results of histopathological features, serological biomarkers, and oxidative damage indicators showed that Nrf2 knockout aggravated renal injury. Besides, Nrf2 deletion decreased the nephroprotective effect of AS-IV by suppressing the pSmad3C/p21 pathway and promoting the pSmad3L/PAI-1 pathway. The experimental results were as we suspected. And we identify for the first time that Nrf2 deficiency increases renal fibrosis from hepatocarcinogenesis and attenuates the therapeutic effects of AS-IV via regulating pSmad3C/3L signal pathway.
Graphical Abstract








Similar content being viewed by others
Data availability
The data and materials that support the findings of this study are available from the corresponding author upon reason able request.
Abbreviations
- AS-IV:
-
Astragaloside IV
- BUN:
-
Blood urea nitrogen
- CCl4 :
-
Carbon tetrachloride
- DEN:
-
Diethylinitrosamine
- DMSO:
-
Dimethyl sulfoxide
- HCC:
-
Hepatocellular carcinoma
- HE:
-
Hematoxylin and eosin
- HRS:
-
Hepatorenal syndrome
- HO-1:
-
Heme oxygenase-1
- Masson:
-
Masson-trichrome staining
- MDA:
-
Malondialdehyde
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- NS:
-
Normal saline
- PAI-1:
-
Plasminogen activator inhibitor-1
- pSmad3C:
-
C-terminally phosphorylated Smad3
- pSmad3L:
-
Linker-phosphorylated Smad3
- Scr:
-
Serum creatinine
- SOD:
-
Superoxide dismutase
- TGF-β1:
-
Transforming growth factor-beta 1
- TβRI:
-
TGF-β type I receptor
- α-SMA:
-
Alpha smooth muscle actin
- β-actin:
-
Beta-actin (β-non-muscle)
References
Aladaileh SH, Hussein OE, Abukhalil MH, Saghir SAM, Bin-Jumah M, Alfwuaires MA, Germoush MO, Almaiman AA, Mahmoud AM (2019) Formononetin upregulates Nrf2/HO-1 signaling and prevents oxidative stress, inflammation, and kidney injury in methotrexate-induced rats. Antioxidants (Basel) 8(10):430
Boutron I, Percie du Sert N, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Hurst V, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, Petersen OH, Rawle F, Reynolds P, Rooney K, Sena ES, Silberberg SD, Steckler T, Würbel H (2020) Reporting animal research: explanation and elaboration for the ARRIVE guidelines 2.0. PLOS Biol 18(7):e3000411
Chen Q, Su Y, Ju Y, Ma K, Li W, Li W (2018) Astragalosides IV protected the renal tubular epithelial cells from free fatty acids-induced injury by reducing oxidative stress and apoptosis. Biomed Pharmacother 108:679–686
Chen X, Wei W, Li Y, Huang J, Ci X (2019) Hesperetin relieves cisplatin-induced acute kidney injury by mitigating oxidative stress, inflammation and apoptosis. Chem Biol Interact 308:269–278
Chu H, Huang C, Gao Z, Dong J, Tang Y, Dong Q (2017) Reduction of ischemic brain edema by combined use of paeoniflorin and astragaloside IV via down-regulating connexin 43. Phytother Res 31(9):1410–1418
Csak T, Bernstein D (2022) Hepatorenal syndrome: pathophysiology. Clin Liver Dis 26(2):165–179
Cui X, Jiang X, Wei C, Xing Y, Tong G (2020) Astragaloside IV suppresses development of hepatocellular carcinoma by regulating miR-150-5p/beta-catenin axis. Environ Toxicol Pharmacol 78:103397
Deng HF, Yue LX, Wang NN, Zhou YQ, Zhou W, Liu X, Ni YH, Huang CS, Qiu LZ, Liu H, Tan HL, Tang XL, Wang YG, Ma ZC, Gao Y (2020) Mitochondrial iron overload-mediated inhibition of Nrf2-HO-1/GPX4 assisted ALI-induced nephrotoxicity. Front Pharmacol 11:624529
Ding T, Zhao T, Li Y, Liu Z, Ding J, Ji B, Wang Y, Guo Z (2021) Vitexin exerts protective effects against calcium oxalate crystal-induced kidney pyroptosis in vivo and in vitro. Phytomedicine 86:153562
Fan Y, Cheng J, Yang Q, Feng J, Hu J, Ren Z, Yang H, Yang D, Ding G (2021) Sirt6-mediated Nrf2/HO-1 activation alleviates angiotensin II-induced DNA DSBs and apoptosis in podocytes. Food Funct 12(17):7867–7882
Francoz C, Durand F, Kahn JA, Genyk YS, Nadim MK (2019) Hepatorenal syndrome. Clin J Am Soc Nephrol 14(5):774–781
Fu C, Wu Y, Liu S, Luo C, Lu Y, Liu M, Wang L, Zhang Y, Liu X (2022) Rehmannioside A improves cognitive impairment and alleviates ferroptosis via activating PI3K/AKT/Nrf2 and SLC7A11/GPX4 signaling pathway after ischemia. J Ethnopharmacol 289:115021
Gao P, Du X, Liu L, Xu H, Liu M, Guan X, Zhang C (2020) Astragaloside IV alleviates tacrolimus-induced chronic nephrotoxicity via p62-Keap1-Nrf2 pathway. Front Pharmacol 11:610102
Gong Y, Li D, Li L, Yang J, Ding H, Zhang C, Wen G, Wu C, Fang Z, Hou S, Yang Y (2021) Smad3 C-terminal phosphorylation site mutation attenuates the hepatoprotective effect of salvianolic acid B against hepatocarcinogenesis. Food Chem Toxicol 147:111912
Hama T, Nakanishi K, Sato M, Mukaiyama H, Togawa H, Shima Y, Miyajima M, Nozu K, Nagao S, Takahashi H, Sako M, Iijima K, Yoshikawa N, Suzuki H (2017) Aberrant Smad3 phosphoisoforms in cyst-lining epithelial cells in the cpk mouse, a model of autosomal recessive polycystic kidney disease. Am J Physiol Renal Physiol 313(6):F1223–F1231
Jiang N, Zhao H, Han Y, Li L, Xiong S, Zeng L, Xiao Y, Wei L, Xiong X, Gao P, Yang M, Liu Y, Sun L (2020) HIF-1alpha ameliorates tubular injury in diabetic nephropathy via HO-1-mediated control of mitochondrial dynamics. Cell Prolif 53(11):e12909
Kanlaya R, Peerapen P, Nilnumkhum A, Plumworasawat S, Sueksakit K, Thongboonkerd V (2020) Epigallocatechin-3-gallate prevents TGF-beta1-induced epithelial-mesenchymal transition and fibrotic changes of renal cells via GSK-3beta/beta-catenin/Snail1 and Nrf2 pathways. J Nutr Biochem 76:108266
Li X, Ramadori P, Pfister D, Seehawer M, Zender L, Heikenwalder M (2021) The immunological and metabolic landscape in primary and metastatic liver cancer. Nat Rev Cancer 21(9):541–557
Lu MC, Zhao J, Liu YT, Liu T, Tao MM, You QD, Jiang ZY (2019) CPUY192018, a potent inhibitor of the Keap1-Nrf2 protein-protein interaction, alleviates renal inflammation in mice by restricting oxidative stress and NF-kappaB activation. Redox Biol 26:101266
Lu H, Sun X, Jia M, Sun F, Zhu J, Chen X, Chen K, Jiang K (2021) Rosiglitazone suppresses renal crystal deposition by ameliorating tubular injury resulted from oxidative stress and inflammatory response via promoting the Nrf2/HO-1 pathway and shifting macrophage polarization. Oxid Med Cell Longev 2021:5527137
Luo M, Yang F, Huang S-X, Kuang Z-P, Luo X-L, Li Y-D, Wu J-N, Xie Y-A (2012) Two-stage model of chemically induced hepatocellular carcinoma in mouse. Oncol Res Featuring Preclinical Clin Cancer Ther 20(11):517–528
Lv Y, Yang Z, Liu L, Li K, He C, Wang Z, Bai W, Guo W, Yu T, Yuan X, Zhang H, Xie H, Yao L, Wang J, Li T, Wang Q, Chen H, Wang E, Xia D, Luo B, Li X, Yuan J, Han N, Zhu Y, Niu J, Cai H, Xia J, Yin Z, Wu K, Fan D, Han G (2019) Early TIPS with covered stents versus standard treatment for acute variceal bleeding in patients with advanced cirrhosis: a randomised controlled trial. Lancet Gastroenterol Hepatol 4(8):587–598
Ma JQ, Zhang YJ, Tian ZK, Liu CM (2021) Bixin attenuates carbon tetrachloride induced oxidative stress, inflammation and fibrosis in kidney by regulating the Nrf2/TLR4/MyD88 and PPAR-gamma/TGF-beta1/Smad3 pathway. Int Immunopharmacol 90:107117
Mahmoud AM, Desouky EM, Hozayen WG, Bin-Jumah M, El-Nahass ES, Soliman HA, Farghali AA (2019) Mesoporous silica nanoparticles trigger liver and kidney injury and fibrosis via altering TLR4/NF-kappaB, JAK2/STAT3 and Nrf2/HO-1 signaling in rats. Biomolecules 9(10):528
Matsuzaki K (2013) Smad phospho-isoforms direct context-dependent TGF-β signaling. Cytokine Growth Factor Rev 24(4):385–399
Metwaly HA, El-Eraky AM, Ibrahim EE, Kandil KK, El-Sayed MA, El-Tabakh NM, Motawea AM, Ali HA, Jabban MZ, Mahmoud ME, Abdelfattah WH, Elmorsy MA, Ghanim AMH (2022) Vanillin attenuates thioacetamide-induced renal assault by direct and indirect mediation of the TGFβ, ERK and Smad signalling pathways in rats. Cell Biochem Funct 40(2):189–202
Murata M, Matsuzaki K, Yoshida K, Sekimoto G, Tahashi Y, Mori S, Uemura Y, Sakaida N, Fujisawa J, Seki T, Kobayashi K, Yokote K, Koike K, Okazaki K (2009) Hepatitis B virus X protein shifts human hepatic transforming growth factor (TGF)-β signaling from tumor suppression to oncogenesis in early chronic hepatitis B. Hepatology 49(4):1203–1217
Rose CF, Amodio P, Bajaj JS, Dhiman RK, Montagnese S, Taylor-Robinson SD, Vilstrup H, Jalan R (2020) Hepatic encephalopathy: novel insights into classification, pathophysiology and therapy. J Hepatol 73(6):1526–1547
Samarghandian S, Azimi-Nezhad M, Farkhondeh T, Samini F (2017) Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomed Pharmacother 87:223–229
Shen X, Wang H, Weng C, Jiang H, Chen J (2021) Caspase 3/GSDME-dependent pyroptosis contributes to chemotherapy drug-induced nephrotoxicity. Cell Death Dis 12(2):186
Shopit A, Niu M, Wang H, Tang Z, Li X, Tesfaldet T, Ai J, Ahmad N, Al-Azab M, Tang Z (2020) Protection of diabetes-induced kidney injury by phosphocreatine via the regulation of ERK/Nrf2/HO-1 signaling pathway. Life Sci 242:117248
Tan HK, Teng MLP, Soh AYS, Cheo SHY, Fook-Chong S, Goh GBB, Tan CK, Wong GW, Lee GH, Chang JPE (2021) Poor outcomes of cirrhosis due to nonalcoholic steatohepatitis compared with hepatitis B after decompensation with ascites. Am J Gastroenterol 116(7):1437–1446
Tang PM, Zhang YY, Xiao J, Tang PC, Chung JY, Li J, Xue VW, Huang XR, Chong CC, Ng CF, Lee TL, To KF, Nikolic-Paterson DJ, Lan HY (2020) Neural transcription factor Pou4f1 promotes renal fibrosis via macrophage-myofibroblast transition. Proc Natl Acad Sci U S A 117(34):20741–20752
Uddin MJ, Kim EH, Hannan MA, Ha H (2021) Pharmacotherapy against oxidative stress in chronic kidney disease: promising small molecule natural products targeting Nrf2-HO-1 signaling. Antioxidants (Basel) 10(2):258
Wang S-S, Tsai Y-T, Lee S-D, Chen H-T, Lu C-W, Lee F-Y, Jeng J-S, Liu Y-C, Lo K-J (1991) Spontaneous bacterial peritonitis in patients with hepatitis B-related cirrhosis and hepatocellular carcinoma. Gastroenterology 101(6):1656–1662
Wen Y, Liu Y, Huang Q, Liu R, Liu J, Zhang F, Liu S, Jiang Y (2022) Moringa oleifera Lam. seed extract protects kidney function in rats with diabetic nephropathy by increasing GSK-3beta activity and activating the Nrf2/HO-1 pathway. Phytomedicine 95:153856
Wu X, Guan Y, Yan J, Liu M, Yin Y, Duan J, Wei G, Hu T, Weng Y, Xi M, Wen A (2015) ShenKang injection suppresses kidney fibrosis and oxidative stress via transforming growth factor-beta/Smad3 signalling pathway in vivo and in vitro. J Pharm Pharmacol 67(8):1054–1065
Wu C, Chen W, Fang M, Boye A, Tao X, Xu Y, Hou S, Yang Y (2019) Compound Astragalus and Salvia miltiorrhiza extract inhibits hepatocellular carcinoma progression via miR-145/miR-21 mediated Smad3 phosphorylation. J Ethnopharmacol 231:98–112
Wu F, Zhao Y, Shao Q, Fang K, Dong R, Jiang S, Lu F, Luo J, Chen G (2021a) Ameliorative effects of osthole on experimental renal fibrosis in vivo and in vitro by inhibiting IL-11/ERK1/2 signaling. Front Pharmacol 12:646331
Wu Y, Xiao W, Pei C, Wang M, Wang X, Huang D, Wang F, Wang Z (2021) Astragaloside IV alleviates PM2.5-induced lung injury in rats by modulating TLR4/MyD88/NF-κB signalling pathway. Int Immunopharmacol 91:107290
Xiao L, Xu X, Zhang F, Wang M, Xu Y, Tang D, Wang J, Qin Y, Liu Y, Tang C, He L, Greka A, Zhou Z, Liu F, Dong Z, Sun L (2017) The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol 11:297–311
Yan W, Xu Y, Yuan Y, Tian L, Wang Q, Xie Y, Shao X, Zhang M, Ni Z, Mou S (2017) Renoprotective mechanisms of Astragaloside IV in cisplatin-induced acute kidney injury. Free Radic Res 51(7–8):669–683
Younis NN, Elsherbiny NM, Shaheen MA, Elseweidy MM (2020) Modulation of NADPH oxidase and Nrf2/HO-1 pathway by vanillin in cisplatin-induced nephrotoxicity in rats. J Pharm Pharmacol 72(11):1546–1555
Yu WC, Huang RY, Chou TC (2020) Oligo-fucoidan improves diabetes-induced renal fibrosis via activation of Sirt-1, GLP-1R, and Nrf2/HO-1: an in vitro and in vivo study. Nutrients 12(10):3068
Yu X, Xiao Q, Yu X, Cheng Y, Lin H, Xiang Z (2022) A network pharmacology-based study on the mechanism of astragaloside IV alleviating renal fibrosis through the AKT1/GSK-3β pathway. J Ethnopharmacol 297:115535
Zhang C, Li L, Hou S, Shi Z, Xu W, Wang Q, He Y, Gong Y, Fang Z, Yang Y (2021) Astragaloside IV inhibits hepatocellular carcinoma by continually suppressing the development of fibrosis and regulating pSmad3C/3L and Nrf2/HO-1 pathways. J Ethnopharmacol 279:114350
Zhou X, Sun X, Gong X, Yang Y, Chen C, Shan G, Yao Q (2017) Astragaloside IV from Astragalus membranaceus ameliorates renal interstitial fibrosis by inhibiting inflammation via TLR4/NF-кB in vivo and in vitro. Int Immunopharmacol 42:18–24
Acknowledgements
We would like to thank all members of the Research Center of Anhui Medical University for their effective techniques and valuable help during the experiments. We thank Dr. K. Matsuzaki for presenting us with rabbit polyclonal anti-pSmad3L (Ser208/213) antibody. We thank Professor Xiaoming Meng for the gift of HK-2 cells.
Funding
This study was supported by the National Natural Science Foundation of China (81874354, 82074073).
Author information
Authors and Affiliations
Contributions
QW and XJC conceived and designed the research. QW conducted experiments and wrote the manuscript. MM L and YQ C contributed to new reagents and analytical tools. YY X, LL L and YF G provided the methodology and analyzed data. YY were responsible for the supervision and funding acquisition. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics approval
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Animal Ethics Committee of Anhui Medical University (Ethics approval code: LLSC, 20221064, Approval Date: 2 June 2022).
Consent to participate
Not applicable.
Consent for publication
All authors approved the manuscript to be published.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Q., Xu, J., Li, M. et al. Nrf2 knockout attenuates the astragaloside IV therapeutic effect on kidney fibrosis from liver cancer by regulating pSmad3C/3L pathways. Naunyn-Schmiedeberg's Arch Pharmacol 397, 1687–1700 (2024). https://doi.org/10.1007/s00210-023-02711-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02711-2