Abstract
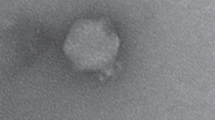
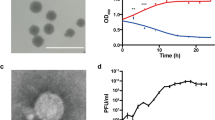
Potato common scab caused by Streptomyces scabies is one of the most economically important diseases infecting potato. It reduces the quality of potato tubers, which subsequently decreases the tuber prices and causes significant economic losses for potato growers. Biological control using bacteriophages is a promising strategy for controlling this disease. In this study, a novel bacteriophage with high lytic efficacy against S. scabies was isolated from a potato field at El-Minya, Egypt, and was designated SscP1EGY. The phage has an icosahedral head of 55 nm and a short tail of 7.5 nm, typical of a podovirus. Its infection cycle was 90 min, including 50 min of latent time and 40 min of rise period with a burst size of approximately 200 PFU per infected cell. The genome of SscP1EGY consists 51,751 nucleotides with 76 predicted genes. SscP1EGY infected and completely lysed seven tested S. scabies strains but showed no lytic activity against three beneficial Streptomyces species, other beneficial bacterial species, and non-target plant pathogenic bacteria. In greenhouse experiments, treatment of S. scabies-inoculated potato tubers with phage SscP1EGY resulted in reductions of (1) the severity of scab, (2) the number of lesions, and (3) the percentage of lesion surface, as compared to the inoculated tubers without phage treatment. Also, scab lesions appeared superficial in phage-treated tubers but pitted in non-phage-treated tubers. Our results suggest that SscP1EGY has a potential as a biological control agent for S. scabies. Based on our knowledge, SscP1EGY is the first sequenced S. scabies-infecting phage in Egypt.







Similar content being viewed by others
References
Adams M (1959) Bacteriophages. Interscience Publishers, New York
Addy HS, Ahmad AA, Huang Q (2019) Molecular and biological characterization of Ralstonia phage RsoM1USA, a new species of P2virus, isolated in the United States. Front Microbiol. https://doi.org/10.3389/fmicb.2019.00267
Adriaenssens E, Brister JR (2017) How to name and classify your phage: an informal guide. Viruses 9(4):70. https://doi.org/10.3390/v9040070
Ahmad AA, Stulberg MJ, Mershon JP, Mollov DS, Huang Q (2017) Molecular and biological characterization of ϕRs551, a filamentous bacteriophage isolated from a race 3 biovar 2 strain of Ralstonia solanacearum. PLoS ONE 12:e0185034
Ahmad AA, Elhalag KM, Addy HS, Nasr-Eldin MA, Hussien AS, Huang Q (2018) Sequencing, genome analysis and host range of a novel Ralstonia phage, RsoP1EGY, isolated in Egypt. Arch Virol 163(8):2271–2274
Alič Š, Naglič T, Tušek-Žnidarič M, Ravnikar M, Rački N, Peterka M, Dreo T (2017) Newly isolated bacteriophages from the Podoviridae, Siphoviridae, and Myoviridae families have variable effects on putative novel Dickeya spp. Front Microbiol 8:1870
Alkhazindar M, Sayed ETA, Khalil MS, Zahran D (2016) Isolation and characterization of two phages infecting Streptomyces scabies. Res J Pharm Biol Chem Sci 7:1351–1363
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Arndt D, Grant JR, Marcu A, Sajed T, Pon A, Liang Y, Wishart DS (2016) PHASTER: a better, faster version of the PHAST phage search tool. Nucleic Acids Res 44:W16–W21
Balogh B, Jones JB, Iriarte FB, Momol MT (2010) Phage therapy for plant disease control. Curr Pharm Biotechnol 11:48–57
Besemer J (2001) GeneMarkS: a self-training method for prediction of gene starts in microbial genomes. Implications for finding sequence motifs in regulatory regions. Nucleic Acids Res 29:2607–2618
Bouchek-Mechiche K, Pasco C, Andrivon D, Jouan B (2000) Differences in host ranges, pathogenicity to potato cultivars and response to soil temperature among Streptomyces species causing common and netted scab in France. Plant Pathol 49:3–10
Buttimer C, McAuliffe O, Ross RP, Hill C, O’Mahony J, Coffey A (2017) Bacteriophages and bacterial plant diseases. Front Microbiol 8:34. https://doi.org/10.3389/fmicb.2017.00034
Davis JR, Sorensen LH (1986) Effects of soil solarisation with moderate air temperatures on potato production. Am Potato J 65:520
Dees MW, Wanner LA (2012) In search of better management of potato common scab. Potato Res 55:249–268
Driscoll J, Joseph C, Ray H, Kirk W, Leslie W, David D (2009) Greenhouse and field nursery evaluation for potato common scab tolerance in a tetraploid population. Am J Potato Res 86:96–101
Elhalag K, Nasr-Eldin M, Hussien A, Ahmad A (2018) Potential use of soilborne lytic Podoviridae phage as a biocontrol agent against Ralstonia solanacearum. J Basic Microbiol 58:658–669
El-Sayed ES, El-Didamony G, Mansour K (2001) Isolation and characterization of two types of actinophage infecting Streptomyces scabies. Folia Microbiol 46:519–526
Elsharouny THM (2007) Studies on bacterial viruses (Bacteriophages) of certain bacteria contributing to soil fertility. M. Sc. Thesis. Dept. Agric. Microbiol. Fac. Agric. Minia University, Egypt.
Fouchard S, Abdellaoui-Maane Z, Boulanger A, Llopiz P, Neunlist S (2005) Influence of growth conditions on Pseudomonas fluorescens strains: a link between metabolite production and the PLFA profile. FEMS Microbiol Lett 251:211–218
Frampton RA, Andrew RP, Peter CF (2012) Advances in bacteriophage-mediated control of plant pathogens. Int J Microbioly. https://doi.org/10.1155/2012/326452
Francki RIB, Fauquet CM, Knudson DL, Brown F (1991) Classification and nomenclature of viruses. Fifth report of the international committee on taxonomy of viruses. Arch Virology Suppl 2:161–166
Gasic K, Ivanovic MM, Ignjatov M, Calic A, Obradovic A (2011) Isolation and characterization of Xanthomonas euvesicatoria bacteriophages. J Plant Pathol 93:415–423
Goyer C (2005) Isolation and characterization of phages Stsc1 and Stsc3 infecting Streptomyces scabiei and their potential as biocontrol agents. Can J Plant Pathol 27:210–216
Hamdi S, Rousseau GM, Labrie SJ, Kourda RS (2016) Characterization of five Podoviridae phages infecting Citrobacter freundii. Front Microbiol 7:1023
Hayashida S, Choi MY, Nanri N, Yokoyama M, Uematsu T (1989) Control of potato common scab with an antibiotic biofertilizer produced from swine feces containing Streptomyces albidoflavus CH-33. Agr Biol Chem 53:349–354
Hayat MA, Miller SE (1990) Negative staining. McGraw-Hill Publishing Co
Hayward AC (2000) Ralstonia solanacearum. In: Lederberg J (ed) Encyclopedia of microbiology, vol 4. Academic Preess, San Diego, pp 32–42
Hooker WJ (1981) Common scab. In: Hooker WJ (ed) Compendium of potato diseases. St Paul. 33–34
Jones JB, Jackson LE, Balogh B, Obradovic A, Iriarte FB, Momol MT (2007) Bacteriophages for plant disease control. Annu Rev Phytopathol 45:245–262
Khan N, Pilar M, Tyler AI, Maskit M, Ethan AH, Najmeh N, Erin RS, Drora K, Ann MH (2018) Antifungal activity of Bacillus species against Fusarium and analysis of the potential mechanisms used in biocontrol. Front Microbiol 9:2363
Klement Z, Rudolf K, Sands DC (1990) Methods in phytobacteriology. Akadémiai Kiadó, Budapest
Lapwood DH, Adams MJ (1975) Mechanisms of control of common scab by irrigation. In: Bruehl GW (ed) Biology and Control of Soil-Borne Pathogens. St Paul. P 123–9
Lazarovits G, Conn KL, Potter J (1999) Reduction of potato scab, Verticillium wilt, and nematodes by soymeal and meat and bone meal in two Ontario potato fields. Can J Plant Pathol 21:345–353
Lee DH, Lim JA, Lee J et al (2013) Characterization of genes required for the pathogenicity of Pectobacterium carotovorum subsp. carotovorum Pcc21 in Chinese cabbage. Microbiol 159(7):1487–1496
Li H, Parent LE, Tremblay G, Karam A (1999) Potato response to crop sequence and nitrogen fertilization following sod breakup in a Gleyed Humo-Ferric Podzol. Can J Plant Sci 79:439–446
Loria R, Kempter BA (1986) Relative resistance of potato tubers produced from stem cuttings and seed-piece-propagated plants to Streptomyces scabies. Plant Dis 7012:1146–1148
Loria R, Bukhalid RA, Fry BA, King RR (1997) Plant pathogenicity in the genus Streptomyces. Plant Dis 81:836–846
Marsh P, Wellington EMH (1994) Phage–host interactions in soil. FEMS Microbiol Ecol 15:99–108
McIntosh AH, Bateman GL, Chamberlain K (1988) Substituted benzoic and picolinic acids as foliar sprays against potato common scab. Ann Appl Biol 112:397–401
McKenna F, El-Tarabily KA, Hardy GESTJ, Dell B (2001) Novel in vivo use of a polyvalent Streptomyces phage to disinfest Streptomyces scabies-infected seed potatoes. Plant Pathol 50:666–675
Mishra BB, Srivastav JS (1996) Effect of pH on the common scab disease of potato. Environ Ecol 14:387–389
Neeno-Eckwall EC, Kinkel LL, Schottel JL (2001) Competition and antibiosis in the biological control of potato scab. Can J Microbiol 47:332–340
Nouioui I, Carro L, García-López M, Meier-Kolthoff JP, Woyke T et al (2018) Genome-based taxonomic classification of the phylum Actinobacteria. Front Microbiol 9:2007
Ogiso H, Akino S, Ogoshi A (1999) Identification of two types of actinophages parasitic to potato common scab pathogens in Hokkaido, Japan. Soil Microorg 53:37–43
Pacífico C, Miriam H, Dmitrij S, Nora D, Ildiko-Julia P, Christoph A, João A, Friederike H (2019) Natural occurrence of Escherichia coli-infecting bacteriophages in clinical samples. Front Microbiol 10:2484
Pringsulaka O, Patarasinpaiboon N, Suwannasai N, Atthakor W, Rangsiruji A (2011) Isolation and characterisation of a novel Podoviridae-phage infecting Weissella cibaria N 22 from Nham, a thai fermented pork sausage. Food Microbiol 28:518–525
Raju K, Naik MK (2006) Effect of pre-harvest spray of fungicides and botanicals on storage diseases of onion. Indian Phytopathol 59:133–141
Raupach GS, Liu L, Morphy JF, Tuzun S, Kloepper JW (1996) Induced systemic resistance in cucumber and tomato against cucumber mosaic cucumovirus using plant growth-promoting rhizobacteria (PGPR). Plant Dis 80:891–894
Salam N, Jiao JY, Zhang XT, Li WJ (2020) Update on the classification of higher ranks in the phylum Actinobacteria. Int J Syst Evol Microbiol 70(2):1331–1355. https://doi.org/10.1099/ijsem.0.003920
Sambrook J, Russell DW (2001) Extraction of bacteriophage λ DNA from large-scale cultures using proteinase K and SDS. In: Sambrook J, Russell DW (eds) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York, pp 256–258
Schmiedeknecht G, Bochow H, Junge H (1998) Use of Bacillus subtilis as biocontrol agent. II. Biological control of potato diseases. J Plant Dis Prot 105:376–386
Stacey G, Pocratsky LA, Puvanesarajah V (1984) Bacteriophage that can distinguish between wild type Rhizobium japonicum and a non-nodulating mutant. Appl Environ Microbiol 48:68–72
Svircev M, Castle AJ, Lehman SM (2010) Bacteriophages for control of phytopathogens in food production systems. In: Sabourand PM, Griffiths MW (eds) bacteriophages in the control of food- and waterborne pathogens. ASM Press, Washington, pp 79–102
Sykes IK, Lanning S, Williams ST (1981) The effect of pH on soil actinophage. J Gen Microbiol 122:271–280
Taha ME (2000) Soil fertility management in Egypt. Proceedings of the Regional Workshop on Soil fertility Management through Farmer Field Schools in the Near East, 2–5 Oct. 2000, Amman, Jordan
Ushiki J, Tahara S, Hayakawa Y, Tadano T (1998) Medicinal plants for suppressing soilborne plant diseases. II. Suppressive effect of Geranium pratense L. on common scab of potato and identification of the active compound. Soil Sci Plant Nutr 44:157–165
Vidaver AK, Schuster ML (1969) Characterization of Xanthomonas phaseoli bacteriophages. J Virol 4(3):300–308
Wilson CR, Ransom LM, Pemberton BM (1999) The relative importance of seed-borne inoculum to common scab disease of potato and the efficacy of seed tuber and soil treatments for disease control. J Phytopathol 147:13–18
Acknowledgements
We would like to thank Drs. Mohamed Hosny, Dept. of Plant Pathology, and Mazhar El-Isawi, Dept. of Microbiology, Sohag University in Egypt for providing the S. scabies 1 isolate used for isolation of phage SscP1EGY and the three beneficial Streptomyces species used for testing the host range of phage SscP1EGY, respectively.
Author information
Authors and Affiliations
Contributions
ASA, AAA, MO, AH, and QH Conceived and designed the experiments, and contributed to the formal analysis. ASA, MO, and AH performed the experiments. ASA, AAA, and QH wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflicts of interest.
Ethical approval
This study did not include experiments with human participants or animals performed by any of the authors.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abdelrhim, A.S., Ahmad, A.A., Omar, M.O.A. et al. A new Streptomyces scabies-infecting bacteriophage from Egypt with promising biocontrol traits. Arch Microbiol 203, 4233–4242 (2021). https://doi.org/10.1007/s00203-021-02415-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-021-02415-2