Abstract
Purpose
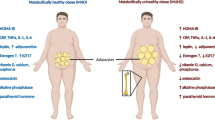
Osteoporosis is a metabolic bone disease characterized by decreased bone strength and mass, which predisposes patients to fractures and is associated with high morbidity and mortality. Like osteoporosis, obesity and diabetes are systemic metabolic diseases associated with modifiable risk factors and lifestyle, and their prevalence is increasing. They are related to decreased quality of life, functional loss and increased mortality, generating high costs for health systems and representing a worldwide public health problem. Growing evidence reinforces the role of bone marrow adipose tissue (BMAT) as an influential factor in the bone microenvironment and systemic metabolism. Given the impact of obesity and diabetes on metabolism and their possible effect on the bone microenvironment, changes in BMAT behavior may explain the risk of developing osteoporosis in the presence of these comorbidities.
Methods
This study reviewed the scientific literature on the behavior of BMAT in pathological metabolic conditions, such as obesity and diabetes, and its potential involvement in the pathogenesis of bone fragility.
Results
Published data strongly suggest a relationship between increased BMAT adiposity and the risk of bone fragility in the context of obesity and diabetes.
Conclusion
By secreting a broad range of factors, BMAT modulates the bone microenvironment and metabolism, ultimately affecting skeletal health. A better understanding of the relationship between BMAT expansion and metabolic disturbances observed in diabetic and obese patients will help to identify regulatory pathways and new targets for the treatment of bone-related diseases, with BMAT as a potential therapeutic target.

Similar content being viewed by others
References
Gkastaris K, Goulis DG, Potoupnis M, Anastasilaki AD, Kapetanos G (2020) Obesity, osteoporosis and bone metabolism. J Musculoskelet Neuronal Interact 20:372–381
Cosman F, Beur SJ, Leboff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R (2014) Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 25:2359–2381. https://doi.org/10.1007/s00198-014-2794-2
Bolland MJ, Grey AB, Gamble GD, Reid IR (2010) Effect of osteoporosis treatment on mortality: a meta-analysis. J Clin Endocrinol Metab 95:1174–1181. https://doi.org/10.1210/jc.2009-0852
World Health Organization (2017) Global Health Observatory. Obesity: Situation and trends. https://www.who.int/health-topics/obesity#tab=tab_1 [acessado em 06 de dezembro de 2021]
Güngör NK (2014) Overweight and obesity in children and adolescents. J Clin Res Pediatr Endocrinol 6:129–143. https://doi.org/10.4274/Jcrpe.1471
Maffi P, Secchi A (2017) The burden of diabetes: emerging data. Dev Ophthalmol 60:1–5. https://doi.org/10.1159/000459641
Premaor MO, Comim FV, Compston JE (2014) Obesity and fractures. Arq Bras de Endocrinol Metab 58:470–477. https://doi.org/10.1590/0004-2730000003274
Barrera G, Bunout D, Gattás V, de la Maza MP, Leiva L, Hirsch S (2004) A high body mass index protects against femoral neck osteoporosis in healthy elderly subjects. Nutrition 20(9):769–771. https://doi.org/10.1016/j.nut.2004.05.014
Kim SJ, Yang WG, Cho E, Park EC (2012) Relationship between weight, body mass index and bone mineral density of lumbar spine in women. J Bone Metab 19(2):95–102. https://doi.org/10.11005/jbm.2012.19.2.95
Ouyang Y, Quan Y, Guo C, Xie S, Liu C, Huang X, Huang X, Chen Y, Xiao X, Ma N, Xie R (2022) Saturation effect of body mass index on bone mineral density in adolescents of different ages: a population-based study. Front Endocrinol (Lausanne) 5(13):922903. https://doi.org/10.3389/fendo.2022.922903
Hong AR, Kim SW (2018) Effects of resistance exercise on bone health. Endocrinol Metab (Seoul) 33(4):435–444. https://doi.org/10.3803/EnM.2018.33.4.435
Robling AG, Castillo AB, Turner CH (2006) Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng 8:455–498. https://doi.org/10.1146/annurev.bioeng.8.061505.095721
Fassio A, Idolazzi L, Rossini M, Gatti D, Adami G, Giollo A, Viapiana O (2018) The obesity paradox and osteoporosis. Eat Weight Disord 23:293–302. https://doi.org/10.1007/s40519-018-0505-2
Cheung YM, Joham A, Marks S, Teede H (2017) The obesity paradox: an endocrine perspective. Intern Med J 47:727–733. https://doi.org/10.1111/imj.13257
De Laet C, Kanis JA, Odén A, Johanson H, Johnell O, Delmas P et al (2005) Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int 16:1330–1338. https://doi.org/10.1007/s00198-005-1863-y
Compston JE, Watts NB, Chapurlat R, Cooper C, Boonen S, Greenspan S et al (2011) Obesity is not protective against fracture in postmenopausal women: glow. Am J Med 124:1043–1050. https://doi.org/10.1016/j.amjmed.2011.06.013
Prieto-Alhambra D, Premaor MO, Fina Avilés F, Hermosilla E, Martinez-Laguna D, Carbonell-Abella C et al (2012) The association between fracture and obesity is site-dependent: A population-based study in postmenopausal women. J Bone Miner Res 27:294–300. https://doi.org/10.1002/jbmr.1466
Rinonapoli G, Pace V, Ruggiero C, Ceccarini P, Bisaccia M, Meccariello L, Caraffa A (2021) Obesity and bone: a complex relationship. Int J Mol Sci 22(24):13662. https://doi.org/10.3390/ijms222413662
Cauley JA (2015) Estrogen and bone health in men and women. Steroids 99:11–15. https://doi.org/10.1016/j.steroids.2014.12.010
Walsh JS, Vilaca T (2017) Obesity, type 2 diabetes and bone in adults. Calcif Tissue Int 100:528–535. https://doi.org/10.1007/s00223-016-0229-0
Scott D, Daly RM, Sanders KM, Ebeling PR (2015) Fall and fracture risk in sarcopenia and dynapenia with and without obesity: the role of lifestyle interventions. Curr Osteoporos Rep 13:235–244. https://doi.org/10.1007/s11914-015-0274-z
Scott D, Chandrasekara SD, Laslett LL, Cicuttini F, Ebeling PR, Jones G (2016) Associations of sarcopenic obesity and dynapenic obesity with bone mineral density and incident fractures over 5–10 years in community-dwelling older adults. Calcif Tissue Int 99:30–42. https://doi.org/10.1007/s00223-016-0123-
Himes CL, Reynolds SL (2012) Effect of obesity on falls, injury, and disability. J Am Geriatr Soc 60:124–129. https://doi.org/10.1111/j.1532-5415.2011.03767.x
Bachmann KN, Fazeli PK, Lawson EA, Russell BM, Riccio AD, Meenaghan E et al (2014) Comparison of hip geometry, strength, and estimated fracture risk in women with anorexia nervosa and overweight/obese women. J Clin Endocrinol Metab 99:4664–4673. https://doi.org/10.1210/jc.2014-2104
Ho-Pham LT, Nguyen ND, Lai TQ, Nguyen TV (2010) Contributions of lean mass and fat mass to bone mineral density: a study in postmenopausal women. Musculoskelet Disord 26(11):59. https://doi.org/10.1186/1471-2474-11-59
Soininen S, Sidoroff V, Lindi V, Mahonen A, Kröger L, Kröger H, Jääskeläinen J, Atalay M, Laaksonen DE, Laitinen T, Lakka TA (2018) Body fat mass, lean body mass and associated biomarkers as determinants of bone mineral density in children 6–8years of age - The Physical Activity and Nutrition in Children (PANIC) study. Bone 108:106–114. https://doi.org/10.1016/j.bone.2018.01.003
Kim KC, Shin DH, Lee SY, Im JA, Lee DC (2010) Relation between obesity and bone mineral density and vertebral fractures in Korean postmenopausal women. Yonsei Med J 51(6):857–863. https://doi.org/10.3349/ymj.2010.51.6.857
Jain RK, Vokes T (2022) Fat mass has negative effects on bone, especially in men: a cross-sectional analysis of NHANES 2011–2018. J Clin Endocrinol Metab 107(6):e2545–e2552. https://doi.org/10.1210/clinem/dgac040
Zhao LJ, Jiang H, Papasian CJ, Maulik D, Drees B, Hamilton J, Deng HW (2008) Correlation of obesity and osteoporosis: Effect of fat mass on the determination of osteoporosis. J Bone Miner Res 23:17–29. https://doi.org/10.1359/jbmr.070813
Bogl LH, Latvala A, Kaprio J, Sovijärvi O, Rissanen A, Pietiläinen KH (2011) An investigation into the relationship between soft tissue body composition and bone mineral density in a young adult twin sample. J Bone Miner Res 26(1):79–87. https://doi.org/10.1002/jbmr.192
Kurra S, Fink DA, Siris ES (2014) Osteoporosis-associated fracture and diabetes. Endocrinol Metab Clin North Am 43:233–243. https://doi.org/10.1016/j.ecl.2013.09.004
Cortet B, Lucas S, Legroux-Gerot I, Penel G, Chauveau C, Paccou J (2019) Bone disorders associated with diabetes mellitus and its treatments. Joint Bone Spine 86:315–320. https://doi.org/10.1016/j.jbspin.2018.08.002
Sealand R, Razavi C, Adler RA (2013) Diabetes mellitus and osteoporosis. Curr DiabRep 13:411–418. https://doi.org/10.1007/s11892-013-0376-x
Cavati G, Pirrotta F, Merlotti D, Ceccarelli E, Calabrese M, Gennari L, Mingiano C (2023) Role of advanced glycation end-products and oxidative stress in Type-2-diabetes-induced bone fragility and implications on fracture risk stratification. Antioxidants (Basel) 12(4):928. https://doi.org/10.3390/antiox12040928
Tl W, Voziyan P, Nyman JS (2022) Causative or associative: A critical review of the role of advanced glycation end-products in bone fragility. Bone 163:116485. https://doi.org/10.1016/j.bone.2022.116485
Sanguineti R, Puddu A, Mach F, Montecucco F, Viviani GL (2014) Advanced glycation end products play adverse proinflammatory activities in osteoporosis. Mediators Inflamm 2014:975872. https://doi.org/10.1155/2014/975872
Paschou SA, Dede AD, Anagnostis PG, Vryonidou A, Morganstein D, Goulis DG (2017) Type 2 diabetes and osteoporosis: A guide to optimal management. J Clin Endocrinol Metab 102:3621–3634. https://doi.org/10.1210/jc.2017-00042
Li Y, Meng Y, Yu X (2019) The unique metabolic characteristics of bone marrow adipose tissue. Front Endocrinol 10:69. https://doi.org/10.3389/fendo.2019.00069
Cawthorn WP, Scheller EL, Learman BS, Parlee SD, Simon BR, Mori H et al (2014) Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab 20:368–375. https://doi.org/10.1016/j.cmet.2014.06.003
Suchacki KJ, Tavares AAS, Mattiucci D, Scheller EL, Papanastasiou G, Gray C et al (2020) Bone marrow adipose tissue is a unique adipose subtype with distinct roles in glucose homeostasis. Nat Commun 11:3097. https://doi.org/10.1038/s41467-020-168782
Pham TT, Ivaska KK, Hannukainen JC, Virtanen KA, Lidell ME, Enerbäck S, Mäkelä K, Parkkola R, Piirola S, Oikonen V, Nuutila P, Kiviranta R (2020) Human bone marrow adipose tissue is a metabolically active and insulin-sensitive distinct fat depot. J Clin Endocrinol Metab 105(7):2300–10. https://doi.org/10.1210/clinem/dgaa216
Tavassoli M (1976) Marrow adipose cells. Histochemical identification of labile and stable components. Arch Pathol Lab Med 100(1):16–18
Scheller EL, Doucette CR, Learman BS, Cawthorn WP, Khandaker S, Schell B et al (2015) Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat Commun 6:7808. https://doi.org/10.1038/ncomms8808
Li Z, Hardij J, Bagchi DP, Scheller EL, Macdougald OA (2018) Development, regulation, metabolism and function of bone marrow adipose tissues. Bone 110:134–140. https://doi.org/10.1016/j.bone.2018.01.008
Bredella MA, Buckless C, Fazeli PK, Rosen CJ, Torriani M, Klibanski A, Miller KK (2021) Bone marrow adipose tissue composition following high-caloric feeding and fasting. Bone. https://doi.org/10.1016/j.bone.2021.116093
Beekman KM, Regenboog M, Nederveen AJ, Bravenboer N, Den Heijer M, Bisschop PH, Hollak CE, Akkerman EM, Maas M (2022) Gender- and age-associated differences in bone marrow adipose tissue and bone marrow fat unsaturation throughout the skeleton, quantified using chemical shift encoding-based water-fat MRI. Front Endocrinol (Lausanne) 27(13):815–835. https://doi.org/10.3389/fendo.2022.815835
Patsch JM, Li X, Baum T, Yap SP, Karampinos DC, Schwartz AV, Link TM (2013) Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. J Bone Miner Res 28(8):1721–1728. https://doi.org/10.1002/jbmr.1950
Piotrowska K, Tarnowski M (2021) Bone marrow adipocytes-role in physiology and various nutritional conditions in human and animal models. Nutrients 13(5):1412. https://doi.org/10.3390/nu13051412
Schoettl T, Fischer IP, Ussar S (2018) Heterogeneity of adipose tissue in development and metabolic function. J Exp Biol. https://doi.org/10.1242/jeb.162958
Hildebrand S, Stümer J, Pfeifer A (2018) PVAT and its relation to brown, beige, and white adipose tissue in development and function. Front Physiol 9:70. https://doi.org/10.3389/fphys.2018.00070
Pellegrinelli V, Carobbio S, Vidal-Puig A (2016) Adipose tissue plasticity: how fat depots respond differently to pathophysiological cues. Diabetologia 59:1075–1088. https://doi.org/10.1007/s00125-016-3933-4
Zhong L, Yao L, Tower Rj, Wei Y, Miao Z, Park J (2020) Single cell transcriptomics identifies a unique adipose lineage cell population that regulates bone marrow environment. eLife 9. https://doi.org/10.7554/eLife.54695
Herrmann M (2019) Marrow fat-secreted factors as biomarkers for osteoporosis. Curr Osteoporos Rep 17:429–437. https://doi.org/10.1007/s11914-019-00550-w
Sulston RJ, Cawthorn WP (2016) Bone marrow adipose tissue as an endocrine organ: close to the bone? Horm Mol Biol Clin Investig 28:21–38. https://doi.org/10.1515/hmbci-2016-0012
Liu LF, Shen WJ, Ueno M, Patel S, Kraemer FB (2011) Characterization of age-related gene expression profiling in bone marrow and epididymal adipocytes. BMC Genomics 12:212. https://doi.org/10.1186/1471-2164-12-212
Yu W, Zhong L, Yao L, Wei Y, Gui T, Li Z (2021) Bone marrow adipogenic lineage precursors promote osteoclastogenesis in bone remodeling and pathologic bone loss. J Clin Invest 131. https://doi.org/10.1172/JCI140214
Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B (2012) Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone 50:546–552. https://doi.org/10.1016/j.bone.2011.06.016
Lecka-Czernik B, Stechschulte LA, Czernik PJ, Sherman SB, Huang S, Krings A (2017) Marrow adipose tissue: skeletal location, sexual dimorphism, and response to sex steroid deficiency. Front Endocrinol 8:188. https://doi.org/10.3389/fendo.2017.00188
Vander Wyst KB, Hu HH, Peña A, Olson ML, Bailey SS, Shaibi GQ (2021) Bone marrow adipose tissue content in Latino adolescents with prediabetes and obesity. Obesity (Silver Spring) 29(12):2100–2107. https://doi.org/10.1002/oby.23279
Bertheau RC, Lorbeer R, Nattenmüller J, Wintermeyer E, Machann J, Linkohr B, Peters A, Bamberg F, Schlett CL (2020) Bone marrow fat fraction assessment in regard to physical activity: KORA FF4-3-T MR imaging in a population-based cohort. Eur Radiol 30(6):3417–3428. https://doi.org/10.1007/s00330-019-06612-y
Li Z, Frey JL, Wong GW, Faugere MC, Wolfgang MJ, Kin JK et al (2016) Glucose transporter-4 facilitates insulin-stimulated glucose uptake in osteoblasts. Endocrinology 157:4094–4103. https://doi.org/10.1210/en.2016-1583
Qiang G, Whang Kong H, Xu S, Pham HA, Parlee SD, Burr AA et al (2016) Lipodystrophy and severe metabolic dysfunction in mice with adipose tissue-specific insulin receptor ablation. Mol Metab 5:480–490. https://doi.org/10.1016/j.molmet.2016.05.005
Kricun ME (1985) Red-yellow marrow conversion: Its effect on the location of some solitary bone lesions. Skeletal Radiol 14:10–19. https://doi.org/10.1007/BF00361188
Scheller EL, Cawthorn WP, Burr AA, Horowitz MC, Macdougald OA (2016) Marrow adipose tissue: trimming the fat. Trends Endocrinol Metab 27:392–403. https://doi.org/10.1016/j.tem.2016.03.016
Solis-Herrera C, Triplitt C, Cersosimo E, Defronzo RA, Feingold KR, Anawalt B et al (2000) Pathogenesis of Type 2 Diabetes Mellitus, Endotext (Internet). South Dartmouth
Thrailkill KM, Lumpkin CK Jr, Bunn RC, Kemp SF, Fowlkes JL (2005) Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues. Am J Physiol Endocrinol Metab 289:735745. https://doi.org/10.1152/ajpendo.00159.2005
Dennison EM, Syddall HE, Aihie Sayer A, Craighead S, Phillips DI, Cooper C (2004) Type 2 diabetes mellitus is associated with increased axial bone density in men and women from the Hertfordshire Cohort Study: evidence for an indirect effect of insulin resistance? Diabetologia 47:1963–1968. https://doi.org/10.1007/s00125-004-1560-y
Shanbhogue VV, Finkelstein JS, Bouxsein ML, Yu EW (2016) Association between insulin resistance and bone structure in nondiabetic postmenopausal women. J Clin Endocrinol Metab 101:3114–3122. https://doi.org/10.1210/jc.2016-1726
Ermetici F, Briganti S, Delnevo A, Cannaó P, Leo GD, Benedini S et al (2018) Bone marrow fat contributes to insulin sensitivity and adiponectin secretion in premenopausal women. Endocrine 59:410–418. https://doi.org/10.1007/s12020-017-1349-7
De Paula FJA, De Araújo IM, Carvalho AL, Elias J Jr, Salmon CE, Nogueira- Barbosa MH (2015) The relationship of fat distribution and insulin resistance with lumbar spine bone mass in women. PLoS ONE 10. https://doi.org/10.1371/journal.pone.0129764
Ambrosi TH, Schulz TJ (2017) The emerging role of bone marrow adipose tissue in bone health and dysfunction. J Mol Med (Berl) 95(12):1291–1301. https://doi.org/10.1007/s00109-017-1604-7
Li Z, Rosen CJ (2023) The multifaceted roles of bone marrow adipocytes in bone and hematopoietic homeostasis. J Clin Endocrinol Metab 14:dgad355. https://doi.org/10.1210/clinem/dgad355
Durdan MM, Azaria RD, Weivoda MM (2022) Novel insights into the coupling of osteoclasts and resorption to bone formation. Semin Cell Dev Biol 123:4–13. https://doi.org/10.1016/j.semcdb.2021.10.008
Onji M, Werschler N, Penninger J (2021) A critical relationship between bone and fat: the role of bone marrow adipose‐derived RANKL in bone metabolism. EMBO Rep 22. https://doi.org/10.15252/embr.202152986
Sekiya I, Larson BL, Vuoristo JT, Cui JG, Prockop DJ (2004) Adipogenic differentiation of human adult stem cells from bone Marrow Stroma (MSCs). J Bone Miner Res 19:256–264. https://doi.org/10.1359/JBMR.0301220
Ge C, Zhao G, Li B, Li Y, Cawthorn WP, Macdougald OA, Franceschi RT (2018) Genetic inhibition of PPARγ S112 phosphorylation reduces bone formation and stimulates marrow adipogenesis. Bone 107:1–9. https://doi.org/10.1016/j.bone.2017.10.023
Schilling T, Küffner R, Klein-Hitpass L, Zimmer R, Jakob F, Schütze N (2008) Microarray analyses of transdifferentiated mesenchymal stem cells. J Cell Biochem 103:413–433. https://doi.org/10.1002/jcb.21415
Nuttall ME, Gimble JM (2004) Controlling the balance between osteoblastogenesis and adipogenesis and the consequent therapeutic implications. Curr Opin Pharmacol 4:290–294. https://doi.org/10.1016/j.coph.2004.03.002
Costa S, Reagan MR (2019) Therapeutic irradiation: consequences for bone and bone marrow adipose tissue. Front Endocrinol (Lausanne) 29(10):587. https://doi.org/10.3389/fendo.2019.00587
Veldhuis-Vlug AG, Rosen CJ (2018) Clinical implications of bone marrow adiposity. J Intern Med 283(2):121–139. https://doi.org/10.1111/joim.12718
Ali D, Tencerova M, Figeac F, Kassem M, Jafari A (2022) The pathophysiology of osteoporosis in obesity and type 2 diabetes in aging women and men: The mechanisms and roles of increased bone marrow adiposity. Front Endocrinol (Lausanne) 15(13):981487. https://doi.org/10.3389/fendo.2022.981487
Liu L, Rosen CJ (2023) New insights into calorie restriction induced bone loss. Endocrinol Metab (Seoul) 38(2):203–213. https://doi.org/10.3803/EnM.2023.1673
Al Saedi A, Chen L, Phu S, Vogrin S, Miao D, Ferland G, Gaudreau P, Duque G (2020) Age-related increases in marrow fat volumes have regional impacts on bone cell numbers and structure. Calcif Tissue Int 107(2):126–134. https://doi.org/10.1007/s00223-020-00700-8
Tencerova M, Figeac F, Ditzel N, Taipaleenmäki H, Nielsen TK, Kassem M (2018) High-fat diet-induced obesity promotes expansion of bone marrow adipose tissue and impairs skeletal stem cell functions in mice. J Bone Miner Res 33(6):1154–1165. https://doi.org/10.1002/jbmr.3408
Theill LE, Boyle WJ, Penninger JM (2002) RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu Rev Immunol 20:795–823. https://doi.org/10.1146/annurev.immunol.20.100301.064753
Hu Y, Li X, Zhi X, Cong W, Huang B, Chen H (2021) RANKL from bone marrow adipose lineage cells promotes osteoclast formation and bone loss. EMBO Rep 22. https://doi.org/10.15252/embr.202152481
Ono T, Hayashi M, Sasaki F, Nakashima T (2020) RANKL biology: Bone metabolism, the immune system, and beyond. Inflamm Regen 40:2. https://doi.org/10.1186/s41232-019-0111-3
Horowitz MC, Berry R, Holtrup B, Sebo Z, Nelson T, Fretz JA et al (2017) Bone marrow adipocytes. Adipocyte 6:193–204. https://doi.org/10.1080/21623945.2017.1367881
Lecka-Czernik B (2012) Marrow fat metabolism is linked to the systemic energy metabolism. Bone 50:534–539. https://doi.org/10.1016/j.bone.2011.06.032
Ramos-Lobo AM, Donato J Jr (2017) The role of leptin in health and disease. Temperature (Austin) 4(3):258–291. https://doi.org/10.1080/23328940.2017.1327003
Ferron M, Lacombe J (2014) Regulation of energy metabolism by the skeleton: Osteocalcin and beyond. Arch Biochem Biophys 561:137–146. https://doi.org/10.1016/j.abb.2014.05.022
Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y et al (1995) Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1:1155–1161. https://doi.org/10.1038/nm1195-1155
Kumar R, Mal K, Razaq MK, Magsi M, Memon MK, Memon S, Afroz MN, Siddiqui HF, Rizwan A (2020) Association of Leptin With Obesity and Insulin Resistance. Cureus 12(12):e12178. https://doi.org/10.7759/cureus.12178
Tian L, Yu X (2015) Lipid metabolism disorders and bone dysfunction-interrelated and mutually regulated (Review). Mol Med Rep 12:783–794. https://doi.org/10.3892/mmr.2015.3472
Laharrague P, Truel N, Fontanilles AM, Corberand JX, Pénicaud L, Casteilla L (2000) Regulation by cytokines of leptin expression in human bone marrow adipocytes. Horm Metab Res 32:381–385. https://doi.org/10.1055/s-2007-978658
Rydén M, Dicker A, Götherström C, Aström G, Tammik C, Arner P, Le Blanc K (2003) Functional characterization of human mesenchymal stem cell-derived adipocytes. Biochem Biophys Res Commun 311:391–397. https://doi.org/10.1016/j.bbrc.2003.10.010
Corre J, Planat-Benard V, Corberand JX, Pénicaud L, Casteilla L, Laharrague P (2004) Human bone marrow adipocytes support complete myeloid and lymphoid differentiation from human CD34 cells. Br J Haematol 127:344–347. https://doi.org/10.1111/j.1365-2141.2004.05198.x
Belaid-Choucair Z, Lepelletier Y, Poncin G, Thiry A, Humblet C, Maachi M et al (2008) Human bone marrow adipocytes block Granulopoiesis through Neuropilin-1-Induced granulocyte colony-stimulating factor inhibition. Stem Cells 26:1556–1564. https://doi.org/10.1634/stemcells.2008-0068
Uchihashi K, Aoki S, Shigematsu M, Kamochi N, Sonoda E, Soejima H et al (2010) Organotypic culture of human bone marrow adipose tissue. Pathol Int 60:259–267. https://doi.org/10.1111/j.1440-1827.2010.02511.x
Upadhyay J, Farr OM, Mantzoros CS (2015) The role of leptin in regulating bone metabolism. Metabolism 64:105–113. https://doi.org/10.1016/j.metabol.2014.10.021
Motyl KJ, Rosen CJ (2012) Understanding leptin-dependent regulation of skeletal homeostasis. Biochimie 94:2089–2096. https://doi.org/10.1016/j.biochi.2012.04.015
Legiran S, Brandi ML (2012) Bone mass regulation of leptin and postmenopausal osteoporosis with obesity. Clin Cases Miner Bone Metab 9(3):145–149
Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G (2000) Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100(2):197–207. https://doi.org/10.1016/s0092-8674(00)81558-5
Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G (2005) Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434(7032):514–520. https://doi.org/10.1038/nature03398
Khosla S (2002) Leptin-central or peripheral to the regulation of bone metabolism? Endocrinology 143(11):4161–4164. https://doi.org/10.1210/en.2002-220843
Díez JJ, Iglesias P (2003) The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol 148:293–300. https://doi.org/10.1530/eje.0.1480293
Ağbaht K, Gürlek A, Karakaya J, Bayraktar M (2009) Circulating adiponectin represents a biomarker of the association between adiposity and bone mineral density. Endocrine 35:371–379. https://doi.org/10.1007/s12020-009-9158-2
Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R (2002) Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun 290:1084–1089. https://doi.org/10.1006/bbrc.2001.6307
Poloni A, Maurizi G, Serrani F, Mancini S, Mc Z, Frontini A, Cinti S et al (2013) Molecular and functional characterization of human boné marrow adipocytes. Exp Hematol 41:558–566. https://doi.org/10.1016/j.exphem.2013.02.005
Berner HS, Lyngstadaas SP, Spahr A, Monjo M, Thommesen L, Drevon CA, Syversen U et al (2004) Adiponectin and its receptors are expressed in bone-forming cells. Bone 35:842–849. https://doi.org/10.1016/j.bone.2004.06.008
Pacheco-Pantoja EL, Waring VJ, Wilson PJ, Fraser WD, Gallagher JA (2013) Adiponectin receptors are present in RANK-L-induced multinucleated osteoclast-like cells. J Recept Signal Transduct Res 33:291–297. https://doi.org/10.3109/10799893.2013.828070
Paik JM, Farwell WR, Taylor EN (2012) Demographic, dietary, and serum factors and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos Int 23:1727–1736. https://doi.org/10.1007/s00198-011-1776-x
Matsuzawa Y, Funahashi T, Kihara S, Shimomura I (2004) Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol 24:29–33. https://doi.org/10.1161/01.ATV.0000099786.99623.EF
Barb D, Williams CJ, Neuwirth AK, Mantzoros CS (2007) Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin 86(3):858S-866S
Naot D, Musson DS, Cornish J (2017) The activity of adiponectin in bone. Calcif Tissue Int 100:486–499. https://doi.org/10.1007/s00223-016-0216-5
Yanai H, Yoshida H (2019) Beneficial effects of adiponectin on glucose and lipid metabolism and atherosclerotic progression: mechanisms and perspectives. Int J Mol Sci 20(5):1190. https://doi.org/10.3390/ijms20051190
Haluzík M, Pařízková J, Haluzík MM (2004) Adiponectin and its role in the obesity-induced insulin resistance and related complications. Physiol Res 53:123–129
Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF (2004) T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci U S A 101:10308–10313. https://doi.org/10.1073/pnas.0403382101
Zhu M, Miura J, Lu LX, Bernier M, Decabo R, Lane MA et al (2004) Circulating adiponectin levels increase in rats on caloric restriction: The potential for insulin sensitization. Exp Gerontol 39:1049–1059. https://doi.org/10.1016/j.exger.2004.03.024
Lenchik L, Register TC, Hsu F-C, Lohman K, Nicklas BJ, Freedman BI et al (2003) Adiponectin as a novel determinant of bone mineral density and visceral fat. Bone 33:646–651. https://doi.org/10.1016/S8756-3282(03)00237-0
Zillikens MC, Uitterlinden AG, Van Leeuwen JP, Berends AL, Henneman P, Van Dijk KW et al (2010) The role of body mass index, insulin, and adiponectin in the relation between fat distribution and bone mineral density. Calcif Tissue Int 86:116–125. https://doi.org/10.1007/s00223-009-9319-6
Gonnelli S, Caffarelli C, Del Santo K, Cadirni A, Guerriero C, Lucani B et al (2008) The relationship of ghrelin and adiponectin with bone mineral density and bone turnover markers in elderly men. Calcif Tissue Int 83:55–60. https://doi.org/10.1007/s00223-008-9149-y
Barbour KE, Zmuda JM, Boudreau R, Strotmeyer ES, Horwitz MJ, Evans RW et al (2011) Adipokines and the risk of fracture in older adults. J Bone Miner Res 26:1568–1576. https://doi.org/10.1002/jbmr.361
Jürimäe J, Rembel K, Jürimäe T, Rehand M (2005) Adiponectin is associated with bone mineral density in perimenopausal women. Horm Metab Res 37:297–302. https://doi.org/10.1055/s-2005-861483
Oshima K, Nampei A, Matsuda M, Iwaki M, Fukuhara A, Hashimoto J et al (2005) Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochem Biophys Res Commun 331:520–526. https://doi.org/10.1016/j.bbrc.2005.03.210
Williams GA, Wang Y, Callon KE, Watson M, Lin JM, Lam JB et al (2009) In vitro and in vivo effects of adiponectin on bone. Endocrinology 150:3603–3610. https://doi.org/10.1210/en.2008-1639
Mitsui Y, Gotoh M, Fukushima N, Shirachi I, Otabe S, Yuan X et al (2011) Hyperadiponectinemia enhances bone formation in mice. BMC Musculoskelet Disord 12:18. https://doi.org/10.1186/1471-2474-12-18
Zhang K, Wang C, Chen Y, Ji X, Chen X, Tian L, Yu X (2015) Preservation of high-fat diet-induced femoral trabecular bone loss through genetic target of TNF-α. Endocrine 50(1):239–249. https://doi.org/10.1007/s12020-015-0554-5
Wang C, Tian L, Zhang K, Chen Y, Chen X, Xie Y et al (2016) Interleukin-6 gene knockout antagonizes high-fat-induced trabecular bone loss. J Mol Endocrinol 57:161–170. https://doi.org/10.1530/JME-16-0076
Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y (2017) Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci 13(4):851–863. https://doi.org/10.5114/aoms.2016.58928
Ferrante AW (2007) Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Inter Med 262:408–414. https://doi.org/10.1111/j.1365-2796.2007.01852.x
Morley JE, Baumgartner RN (2004) Cytokine-related aging process. J Gerontol A Biol Sci Med Sci 59:924–929. https://doi.org/10.1093/gerona/59.9.m924
Ootsuka T, Nakanishi A, Tsukamoto I (2015) Increase in osteoclastogenesis in an obese Otsuka Long-Evans Tokushima fatty rat model. Mol Med Rep 12:3874–3880. https://doi.org/10.3892/mmr.2015.3811
Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL (2005) IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest 115:282–290. https://doi.org/10.1172/JCI23394
Cenci S, Weitzmann MN, Roggia C, Namba N, Novack D, Woodring J et al (2000) Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-α. J Clin Invest 106:1229–1237. https://doi.org/10.1172/JCI11066
Vozarova B, Weyer C, Hanson K, Tataranni PA, Bogardus C, Pratley RE (2001) Circulating Interleukin-6 in relation to adiposity, insulin action, and insulin secretion. Obes Res 9:414–417. https://doi.org/10.1038/oby.2001.54
Faienza MF, D’amato G, Chiarito M, Colaianni G, Colucci S, Grano M et al (2019) Mechanisms involved in childhood obesity-related bone fragility. Front Endocrinol 10:269. https://doi.org/10.3389/fendo.2019.00269
Cooper MS, Walker EA, Bland R, Fraser WD, Hewison M, Stewart PM et al (2000) Expression and functional consequences of 11β-hydroxysteroid dehydrogenase activity in human bone. Bone 27:375–381. https://doi.org/10.1016/S8756-3282(00)00344-6
Tomlinson J, Bujalska I, Stewart PM, Cooper MS (2000) The role of 11 β-Hydroxysteroid dehydrogenase in central obesity and osteoporosis. Endocr Res 26:711–722. https://doi.org/10.3109/07435800009048591
Cooper MS, Bujalska I, Rabbitt E, Walker EA, Bland R, Sheppard MC et al (2001) Modulation of 11β-hydroxysteroid dehydrogenase isozymes by proinflammatory cytokines in osteoblasts: an autocrine switch from glucocorticoid inactivation to activation. J Bone Miner Res 16:1037–1044. https://doi.org/10.1359/jbmr.2001.16.6.1037
Rosen ED, Spiegelman BM (2001) PPARγ: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem 27:37731–37734. https://doi.org/10.1074/jbc.R100034200
Lecka-Czernik B, Moerman EJ, Grant DF, Lehmann JM, Manolagas SC, Jilka RL (2002) Divergent effects of selective peroxisome proliferator-activated Receptor-2 Ligands on adipocyte versus osteoblast differentiation. Endocrinology 143:2376–2384. https://doi.org/10.1210/endo.143.6.8834
Jeon MJ, Kim JA, Kwon SH, Kim SW, Park KS, Park SW et al (2003) Activation of peroxisome proliferator-activated receptor-γ inhibits the Runx2-mediated transcription of osteocalcin in osteoblasts. J Biol Chem 278:23270–23277. https://doi.org/10.1074/jbc.M211610200
Ferré P (2004) The biology of peroxisome proliferator-activated receptors relationship with lipid metabolism and insulin sensitivity. Diabetes 53:43–50. https://doi.org/10.2337/diabetes.53.2007.s43
Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B (2004) Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-γ2 transcription factor and TGF-β/BMP signaling pathways. Aging Cell 3:379–389. https://doi.org/10.1111/j.1474-9728.2004.00127.x
Shockley KR, Lazarenko OP, Czernik PJ, Rosen CJ, Churchill GA, Lecka-Czernik B (2009) PPARγ2 nuclear receptor controls multiple regulatory pathways of osteoblast differentiation from marrow mesenchymal stem cells. J Cell Biochem 106:232–246. https://doi.org/10.1002/jcb.21994
Cho SW, Yang JY, Her SJ, Choi HJ, Jung JY, Sun HJ et al (2011) Osteoblast-targeted overexpression of PPARγ inhibited bone mass gain in male mice and accelerated ovariectomy-induced bone loss in female mice. J Bone Miner Res 26:1939–1952. https://doi.org/10.1002/jbmr.366
Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung U-I, Kubota N et al (2004) PPARγ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest 113:846–855. https://doi.org/10.1172/JCI19900
Mccann MR, Ratneswaran A (2019) The role of PPARγ in childhood obesity-induced fractures. Genes Nutr 27(14):31. https://doi.org/10.1186/s12263-019-0653-7
Chen JR, Lazarenko OP, Wu X, Tong Y, Blackburn ML, Shankar K, Badger TM, Ronis MJ (2010) Obesity reduces bone density associated with activation of PPARγ and suppression of Wnt/β-catenin in rapidly growing male rats. PLoS One 5(10):e13704. https://doi.org/10.1371/journal.pone.0013704
Sharp JC, Copps JC, Liu Q, Ryner LN, Sebastian RA, Zeng GQ et al (2000) Analysis of ovariectomy and estrogen effects on body composition in rats by X-Ray and magnetic resonance imaging techniques. J Bone Miner Res 15:138–146. https://doi.org/10.1359/jbmr.2000.15.1.138
Krishnan V, Bryant HU, Macdougald OA (2006) Regulation of bone mass by Wnt signaling. J Clin Invest 116:1202–1209. https://doi.org/10.1172/JCI28551
Migliaccio S, Greco EA, Fornari R, Donini LM, Lenzi A (2011) Is obesity in women protective against osteoporosis? Diabetes Metab Syndr Obes 4:273–282. https://doi.org/10.2147/DMSO.S11920
Nelson LR, Bulun SE (2001) Estrogen production and action. J Am Acad Dermatol 45(3 Suppl):S116–S124. https://doi.org/10.1067/mjd.2001.117432
Tencerova M, Frost M, Figeac F, Nielsen TK, Ali D, Lauterlein JL et al (2019) Obesity-associated hypermetabolism and accelerated senescence of bone marrow stromal stem cells suggest a potential mechanism for bone fragility. Cell Rep 27:2050–2062. https://doi.org/10.1016/j.celrep.2019.04.066
Cohen A, Dempster DW, Recker RR, Lappe JM, Zhou H, Zwahlen A et al (2013) Abdominal fat is associated with lower bone formation and inferior bone quality in healthy premenopausal women: a transiliac bone biopsy study. J Clin Endocrinol Metab 98:2562–2572. https://doi.org/10.1210/jc.2013-1047
Ng AC, Melton LJ 3rd, Atkinson EJ, Achenbach SJ, Holets MF, Peterson JM et al (2013) Relationship of adiposity to bone volumetric density and microstructure in men and women across the adult lifespan. Bone 55:119–125. https://doi.org/10.1016/j.bone.2013.02.006
Zhang P, Peterson M, Su GL, Wang SC (2015) Visceral adiposity is negatively associated with bone density and muscle attenuation. Am J Clin Nutr 101:337–343. https://doi.org/10.3945/ajcn.113.081778
Caprio M, Infante M, Calanchini M, Mammi C, Fabbri A (2017) Vitamin D: not just the bone. EVIDENCE for beneficial pleiotropic extraskeletal effects. Eat Weight Disord 22:27–41. https://doi.org/10.1007/s40519-016-0312-6
Samuel L, Borrell LN (2013) The effect of body mass index on optimal vitamin D status in U.S. adults: The National Health and Nutrition Examination Survey 2001–2006. Ann Epidemiol 23:409–414. https://doi.org/10.1016/j.annepidem.2013.05.011
Walsh JS, Evans AL, Bowles S, Naylor KE, Jones KS, Schoenmakers I et al (2016) Free 25-hydroxyvitamin D is low in obesity, but there are no adverse associations with bone health. Am J Clin Nutr 103:1465–1471. https://doi.org/10.3945/ajcn.115.120139
Frayling TM et al (2007) A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316(5826):889–894. https://doi.org/10.1126/science.1141634
Guo Y, Liu H, Yang T-L, Li SM, Tian Q, Liu Y-J et al (2011) The fat mass and obesity associated gene, FTO, is also associated with osteoporosis phenotypes. PLoS ONE 6. https://doi.org/10.1371/journal.pone.0027312
Zhang Q, Riddle RC, Yang Q, Rosen CR, Guttridge DC, Dirckx N, Faugere MC, Farber CR, Clements TL (2019) The RNA demethylase FTO is required for maintenance of bone mass and functions to protect osteoblasts from genotoxic damage. Proc Natl Acad Sci U S A 116(36):17980–17989. https://doi.org/10.1073/pnas.1905489116
Proietto J (2020) Obesity and bone. F1000 Res 9:9. https://doi.org/10.12688/f1000research.20875.1
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors GCG, JBCC, JGOS, ACCS, CACS, JMM, LMR and CSOG declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guimarães, G.C., Coelho, J.B.C., Silva, J.G.O. et al. Obesity, diabetes and risk of bone fragility: How BMAT behavior is affected by metabolic disturbances and its influence on bone health. Osteoporos Int 35, 575–588 (2024). https://doi.org/10.1007/s00198-023-06991-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-023-06991-5