Abstract
Summary
Macro- and microarchitectural, bone material property, dynamic histomorphometric, and bone turnover marker data were studied in normal bone mineral density (BMD) post-menopausal women with fragility fracture. Women with fracture had thinner iliac cortices and more homogeneous bone material properties in cortical bone than age/BMD-matched non-fracture women. Low cortical thickness and bone tissue heterogeneity in normal BMD women are associated with prevalent fragility fracture.
Introduction
Bone mass (bone mineral density, (BMD)) of the spine and hip is today’s best single measurement for evaluating future fragility fracture risk. However, the majority of fragility fractures occur in women with BMD T-score above the WHO osteoporotic BMD threshold of − 2.5, indicating that non-BMD endpoints may play a role in their fragility fractures. We hypothesize that in non-osteoporotic women, bone micoarchitecture, bone material properties, dynamic histomorphometric endpoints, and bone turnover markers are related to fragility fracture.
Methods
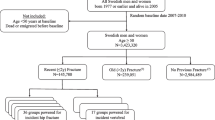
Two groups (N = 60 each) of post-menopausal women with total hip BMD T-score ranging from + 0.3 to –2.49 were recruited: fragility fracture and age/BMD-matched, non-fragility fracture women. Normal (T-score > − 0.99) and osteopenic (T-score ≤ − 1.0) BMD cohorts were designated within both the fracture and non-fracture groups. Transiliac biopsy specimens were obtained to evaluate dynamic histomorphometric and microarchitectural endpoints and bone material properties by static and dynamic nanoindentation testing. All variables for fracture and non-fracture women within each BMD cohort were compared by the Wilcoxon signed-rank test (P < 0.01).
Results
Compared to non-fracture/normal BMD women, fracture/normal BMD women display lower iliac cortical thickness (− 12%, P = 0.0041) and lower heterogeneity of hardness (− 27%, P = 0.0068), elastic modulus (− 35%, P = 0.0009), and storage modulus (− 23%, P = 0.0054) in the cortical bone tissue, and lower heterogeneity of hardness (− 13%, P = 0.0088) in the trabecular bone tissue. Osteopenic women had no abnormalities related to fracture status.
Conclusion
Post-menopausal women with normal BMD and fragility fracture have low cortical thickness and heterogeneity of several bone material properties in cortical and trabecular mineralized bone tissue. These differences may explain a portion of the excess bone fragility in women with normal BMD and fragility fracture.
Similar content being viewed by others
References
Burge R, Dawson-Hughes B et al (2007) Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 22(3):465–475. https://doi.org/10.1359/jbmr.061113
Black DM, Delmas PD, HORIZONPivotalFractureTrial et al (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. New Engl J Med. 356:1809–1822. https://doi.org/10.1056/NEJMoa067312
Hernlund E, Svedbom A, Ivergard M et al (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden. Arch Osteoporos 8(1–2):136. https://doi.org/10.1007/s11657-013-0136-1
Kanis JA, Melton LJ, Christiansen C et al (1994) The diagnosis of osteoporosis. J Bone Min Res 9:1137–1141. https://doi.org/10.1002/jbmr.5650090802
Siris ES, Chen YT, Abbott TA et al (2004) BMD thresholds for pharmacological intervention to prevent fractures. Arch Intern Med 164(10):1108–1112. https://doi.org/10.1001/archinte.164.10.1108
Pasco JA, Seeman E, Henry MJ et al (2006) The population burden of fractures originates in women with osteopenia, not osteoporosis. Osteoporos Int 17(9):1404–1409. https://doi.org/10.1007/s00198-006-0135-9
Nordin BEC (1961) The pathogenesis of osteoporosis. Lancet 1(7185):1011–1015. https://doi.org/10.1016/s0140-6736(61)91827-x
Ross PD, Genant HK, Davis JW et al (1993) Predicting vertebral fracture incidence from prevalent fractures and BMD among non-black, osteoporotic women. Osteo Int 3:120–126. https://doi.org/10.1007/BF01623272
Kanis JA, McCloskey EV, Johansson H et al (2010) Development and use of FRAX in osteoporosis. Osteoporos Int 21(Suppl 2):S407–S413. https://doi.org/10.1007/s00198-010-1253-y
Melton LJ 3rd (1996) Epidemiology of hip fractures: implications of the exponential increase with age. Bone 18(3 Suppl):121S-125S. https://doi.org/10.1016/8756-3282(95)00492-0
Riggs BL, Wahner HW, Dunn WL et al (1981) Differential changes in BMD of the appendicular and axial skeleton with aging: relationship to spinal osteoporosis. J Clin Invest 67(2):328–335. https://doi.org/10.1172/JCI110039
Delmas PD, Seeman E (2004) Changes in BMD explain little of the reduction in vertebral or nonvertebral fracture risk with anti-resorptive therapy. Bone 34:599–604. https://doi.org/10.1016/j.bone.2003.12.022
Van Staa TP, Laan RF, Barton IP et al (2003) BMD threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum 48(11):3224–3229. https://doi.org/10.1002/art.11283
Sellmeyer DE, Civitelli R, Hofbauer LC et al (2016) Skeletal metabolism, fracture risk, and fracture outcomes in Type 1 and Type 2 diabetes. Diabetes 65(7):1757–1766. https://doi.org/10.2337/db16-0063
Frost HM (2002) Emerging views about osteoporosis, bone health, strength, fragility, and their determinants. J Bone Miner Metab 20(6):319–325. https://doi.org/10.1007/s007740200046
Seeman E (2008) Bone quality: the material and structural basis of bone strength. J Bone Miner Metab 26(1):1–8. https://doi.org/10.1007/s00774-007-0793-5
Polly BJ, Yuya PA, Akhter MP et al (2012) Intrinsic material properties of trabecular bone by nanoindentation testing of biopsies taken from healthy women before and after menopause. Calcif Tissue Int 90(4):286–293. https://doi.org/10.1007/s00223-012-9575-8
Uchic MD, Dimiduk DM, Florando JN, Nix WD (2004) Sample dimensions influence strength and crystal plasticity. Science 305(5686):986–989. https://doi.org/10.1126/science.1098993
Vennin S, Desyatova A, Turner JA et al (2017) Intrinsic material property differences in bone tissue from patients suffering low-trauma osteoporotic fractures, compared to matched non-fracturing women. Bone 97:233–242. https://doi.org/10.1016/j.bone.2017.01.031
Rizzo S, Farlay D, Akhter M et al (2018) Variables reflecting the mineralization of bone tissue from fracturing versus non-fracturing post-menopausal nonosteoporotic women. JBMR Plus 2(6):323–327. https://doi.org/10.1002/jbm4.10062
Boskey AL, Donnelly E, Boskey E et al (2015) Examining the relationships between bone tissue composition, compositional heterogeneity and fragility fracture: a matched case controlled FTIRI study. J Bone Miner Res 31(5):1070–1081. https://doi.org/10.1002/jbmr.2759
Oliver WC, Pharr GM (1992) An improved technique for determining hardness and elastic modulus using load displacement sensing indentation experiments. J Mater Res 7(6):1564–1583
Carter DR, Hayes WC (1977) The compressive behavior of bone as a two-phase porous structure. J Bone Joint Surg Am 59(7):954–962
Currey JD (1988) Strain rate and mineral content in fracture models of bone. J Orthop Res 6(1):32–38. https://doi.org/10.1002/jor.1100060105
Cohen SR, Kalfon-Cohen E (2013) Dynamic nanoindentation by instrumented nanoindentation and force microscopy: a comparative review. Beilstein J Nanotechnol 4:815–833. https://doi.org/10.3762/bjnano.4.93
Tai K, Dao M, Suresh S et al (2007) Nanoscale heterogeneity promotes energy dissipation in bone. Nat Mater 6(6):454–462. https://doi.org/10.1038/nmat1911
Akhter MP, Lappe JM, Davies KM, Recker RR (2007) Transmenopausal changes in the trabecular bone structure. Bone 41:111–116. https://doi.org/10.1016/j.bone.2007.03.019
Parfitt AM, Drezner MK, Glorieux FH et al (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR histomorphometry nomenclature committee. J Bone Miner Res 2(6):595–610. https://doi.org/10.1002/jbmr.5650020617
Dempster DW, Compston JE, Drezner MK et al (2013) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 28(1):1–16. https://doi.org/10.1002/jbmr.1805
Kimmel DB, Recker RR, Gallagher JC et al (1990) A comparison of iliac bone histomorphometric data in post-menopausal osteoporotic and normal subjects. Bone Miner 11(2):217–235. https://doi.org/10.1016/0169-6009(90)90061-j
Recker RR, Lappe JM, Davies M, Kimmel D (2018) Perimenopausal bone histomorphometry before and after menopause. Bone 108:55–61. https://doi.org/10.1016/j.bone.2017.12.016
Oleksik A, Ott SM, Vedi S et al (2000) Bone structure in patients with low BMD with or without vertebral fractures. J Bone Miner Res 15(7):1368–1375. https://doi.org/10.1359/jbmr.2000.15.7.1368
Foldes J, Parfitt AM, Shih MS et al (1991) Structural and geometric changes in iliac bone: relationship to normal aging and osteoporosis. J Bone Miner Res 6(7):759–766. https://doi.org/10.1002/jbmr.5650060714
Uitewaal PJM, Lips P, Netelenbos JC (1987) Analysis of bone structure in patients with hip fracture. Bone Mineral 3:63–73
Tarantino U, Rao C, Tempesta V, Gasbarra E, Feola M (2016) Hip fractures in the elderly: The role of cortical bone. Injury 47(Suppl 4):S107–S111. https://doi.org/10.1016/j.injury.2016.07.058
Bousson VD, Adams J, Engelke K et al (2011) In vivo discrimination of hip fracture with quantitative computed tomography: Results from the prospective European Femur Fracture Study (EFFECT). J Bone Miner Res 26(4):881–893. https://doi.org/10.1002/jbmr.270
Yang L, Udall WJ, McCloskey EV, Eastell R (2014) Distribution of bone density and cortical thickness in the proximal femur and their association with hip fracture in postmenopausal women: a quantitative computed tomography study. Osteoporos Int 25(1):251–263. https://doi.org/10.1007/s00198-013-2401-y
Meema HE, Meindok H (1992) Advantages of peripheral radiogrammetry over dual-photon absorptiometry of the spine in the assessment of prevalence of osteoporotic vertebral fractures in women. J Bone Miner Res 7(8):897–903. https://doi.org/10.1002/jbmr.5650070806
Boutroy S, Bouxsein ML, Munoz F, Delmas PD (2005) In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab 90(12):6508–6515. https://doi.org/10.1210/jc.2005-1258
Kral R, Osima M, Borgen TT et al (2017) Increased cortical porosity and reduced cortical thickness of the proximal femur are associated with nonvertebral fracture independent of FRAX and Garvan estimates in postmenopausal women. PLoS ONE 12(9):e0185363. https://doi.org/10.1371/journal.pone.0185363
Sornay-Rendu E, Boutroy S, Duboeuf F, Chapurlat RD (2017) Bone microarchitecture assessed by HR- pQCT as predictor of fracture risk in postmenopausal women: The OFELY Study. J Bone Miner Res 32(6):1243–1251. https://doi.org/10.1002/jbmr.3105
Gourion-Arsiquaud S, Lukashova L, Power J et al (2013) Fourier transform infrared imaging of femoral neck bone: reduced heterogeneity of mineral-to-matrix and carbonate-to-phosphate and more variable crystallinity in treatment-naive fracture cases compared with fracture-free controls. J Bone Miner Res 28(1):150–161. https://doi.org/10.1002/jbmr.1724
Zysset PK, Guo XE, Hoffler CE, Moore KE, Goldstein SA (1999) Elastic modulus and hardness of cortical and trabecular bone lamellae measured by nanoindentation in the human femur. J Biomech 32(10):1005–1012. https://doi.org/10.1016/s0021-9290(99)00111-6
Groetsch A, Gourrier A, Schwiedrzik J et al (2019) Compressive behaviour of uniaxially aligned individual mineralised collagen fibres at the micro- and nanoscale. Acta Biomater 89:313–329. https://doi.org/10.1016/j.actbio.2019.02.053
Tertuliano OA, Greer JR (2016) The nanocomposite nature of bone drives its strength and damage resistance. Nat Mater 15(11):1195–1202. https://doi.org/10.1038/nmat4719
Schwiedrzik J, Raghavan R, Bürki A et al (2014) In situ micropillar compression reveals superior strength and ductility but an absence of damage in lamellar bone. Nat Mater 13(7):740–747. https://doi.org/10.1038/nmat3959
Granke M, Does MD, Nyman JS (2015) The role of water compartments in the material properties of cortical bone. Calcif Tissue Int 97(3):292–307. https://doi.org/10.1007/s00223-015-9977-5
Wolfram U, Wilke HJ, Zysset PK (2010) Rehydration of vertebral trabecular bone: influences on its anisotropy, its stiffness and the indentation work with a view to age, gender and vertebral level. Bone 46(2):348–354. https://doi.org/10.1016/j.bone.2009.09.035
Rokidi S, Bravenboer N, Gamsjaeger S et al (2020) Impact microindentation measurements correlate with cortical bone material properties measured by Fourier transform infrared imaging in humans. Bone 137:115437. https://doi.org/10.1016/j.bone.2020.115437
Mandair GS, Morris MD (2015) Contributions of Raman spectroscopy to the understanding of bone strength. BoneKey 4:1–8. https://doi.org/10.1038/bonekey.2014.115
Arunachalam SP, Rossman PJ, Arani A et al (2017) Quantitative 3D magnetic resonance elastography: Comparison with dynamic mechanical analysis. Magn Reson Med. 77(3):1184–1192. https://doi.org/10.1002/rnrm.26207
Vedi S, Compston JE, Webb A, Tighe JR (1982) Histomorphometric analysis of bone biopsies from the iliac crest of normal British subjects. Metab Bone Dis Relat Res. 4(4):231–6. https://doi.org/10.1016/0221-8747(82)90032-7
Acknowledgements
We acknowledge Susan Bare who collected the histomorphometry data.
Funding
This work was funded by National Institutes of Health Grant # R01 AR054496-01A1 to RRR.
Author information
Authors and Affiliations
Contributions
The following individuals contributed respectively to study design/planning (RRR, MPA, JML, JAT); experimental conduct (RRR, JML); data collection (RRR, JML, SV, AD, MPA); data analysis (DBK, RRR, AD, JAT); data interpretation (all); original draft (DBK, RRR); and revised draft (all).
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kimmel, D.B., Vennin, S., Desyatova, A. et al. Bone architecture, bone material properties, and bone turnover in non-osteoporotic post-menopausal women with fragility fracture. Osteoporos Int 33, 1125–1136 (2022). https://doi.org/10.1007/s00198-022-06308-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-022-06308-y