Abstract
Background and purpose
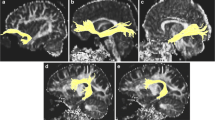
Fixel-based analysis (FBA) is a new method that overcomes the technical limitations of diffusion tensor imaging (DTI) by enabling the characterization of multiple fiber populations within a voxel, and provides biologically meaningful indicators. This study aimed to explore age-related changes in the visual pathway in healthy adults and to observe differences in imaging quality between data collected using different b‑values.
Methods
In this prospective cross-sectional study, brain DTI scans which were collected with more than six uniformly distributed gradient directions and higher b‑values (up to 2000 s/mm2) than traditional DTI were performed in 72 healthy adults across the adult lifespan (20–79 years). After image preprocessing, FBA was used to process the dataset. At the same time, conventional DTI metrics were also calculated.
Results
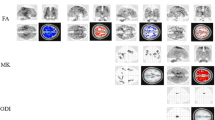
Pearson’s correlation analysis showed that DTI parameters of white matter (optic nerve, optic chiasma, optic tract, and optic radiation) in the optic pathway were correlated with age. FA values were negatively correlated with age, while MD/AD/RD showed a positive correlation (P < 0.05). FBA showed that the index including FD/FC/FDC tended to decline with age (P < 0.05). Linear regression analysis showed a linear relationship between DTI metrics of the dataset collected by b‑values of 1000 and 2000 s/mm2 (P < 0.05).
Conclusion
FBA provides a useful method to assess age-related changes in the visual pathway, which is sensitive to diffusion. In addition, the b‑value influences DTI parameters and signal-to-noise ratio of the image.
Zusammenfassung
Hintergrund und Ziel
Die fixelbasierte Analyse (FBA) ist eine neue Methode, mit der die technischen Begrenzungen der Diffusionstensorbildgebung (DTI) überwindbar werden, da die FBA die Charakterisierung mehrerer Faserpopulationen innerhalb eines Voxels ermöglicht, und so können biologisch bedeutsame Indikatoren erfasst werden. Die vorliegende Studie zielte darauf ab, altersabhängige Veränderungen der Sehbahn bei gesunden Erwachsenen zu untersuchen und Unterschiede in der Bildqualität zwischen Daten, die mit verschiedenen b‑Werten erstellt wurden, zu erkennen.
Methoden
In dieser prospektiven Querschnittstudie wurden DTI-Aufnahmen des Gehirns, die mit mehr als 6 gleichmäßig verteilten Gradientenrichtungen und höheren b‑Werten (bis zu 2000 s/mm2) als herkömmliche DTI-Aufnahmen erstellt wurden, bei 72 gesunden Erwachsenen über die gesamte Lebensspanne von Erwachsenen hinweg (20–79 Jahre) angefertigt. Nach der Bildvorbearbeitung wurde die FBA zur weiteren Datenverarbeitung verwendet. Gleichzeitig wurden auch konventionelle DTI-Messgrößen berechnet.
Ergebnisse
Die Korrelationsanalyse nach Pearson ergab, dass die DTI-Parameter der weißen Substanz (N. opticus, Chiasma opticum, Tractus opticus und Sehstrahlung) in der Sehbahn mit dem Alter korreliert waren. Die FA-Werte waren negativ mit dem Alter korreliert, während mittlere Diffusivität (MD)/axiale Diffusivität (AD)/radiale Diffusivität (RD) eine positive Korrelation aufwiesen (p < 0,05). In der FBA zeigte sich, dass der Index für Faserdichte (FD)/Faserquerschnitt (FC)/Kombination aus FD und FC (FDC) eine abnehmende Tendenz mit dem Alter aufwies (p < 0,05). Die lineare Regressionsanalyse zeigte eine lineare Beziehung zwischen den DTI-Messgrößen des Datensatzes, der mit b‑Werten von 1000 und 2000 s/mm2 erhoben worden war (p < 0,05).
Schlussfolgerung
Die FBA stellt eine hilfreiche Methode zur Bestimmung altersabhängiger Veränderungen in der Sehbahn dar, die empfindlich auf Diffusion reagiert. Darüber hinaus beeinflusst der b‑Wert DTI-Parameter und den Signal-Rausch-Abstand der Aufnahme.





Similar content being viewed by others
References
James D, Swienton AG et al (2014) The visual pathway—functional anatomy and pathology. Semin Ultrasound CT MR 35(5):487–503
Chang EH, Argyelan M, Aggarwal M et al (2017) The role of myelination in measures of white matter integrity: Combination of diffusion tensor imaging and two-photon microscopy of CLARITY intact brains. Neuroimage 147:253–261. https://doi.org/10.1016/j.neuroimage.2016.11.068
Song S‑K, Sun S‑W, Ju W‑K et al (2003) Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 20(3):1714–1722. https://doi.org/10.1016/j.neuroimage.2003.07.005
Haykal S, Curcic-Blake B, Jansonius NM et al (2019) Fixel-based analysis of visual pathway white matter in primary open-angle glaucoma. Investig Ophthalmol Vis Sci. https://doi.org/10.1007/s00234-019-02263-4
Chuck NC, Steidle G, Blume I et al (2013) Diffusion tensor imaging of the kidneys: influence of b‑value and number of encoding directions on image quality and diffusion tensor parameters. J Clin Imaging Sci 2013:3–53. https://doi.org/10.4103/2156-7514.122323
Attyé AMD, Jean CMD, Remond PMD et al (2018) Track-weighted imaging for neuroretina: Evaluations in healthy volunteers and ischemic optic neuropathy. J Magn Reson Imaging. https://doi.org/10.1002/jmri.25941
Ngamsombat C, Wongsawaeng D, Thuangtong A et al (2017) Preoperative diffusion tensor imaging analysis of optic radiations: study in pituitary Macroadenoma patients with optic chiasm compression. J Med Assoc Thai 100(Suppl. 2):S76–S81
Sarlls JE, Pierpaoli C (2009) In vivo diffusion tensor imaging of the human optic chiasm at a sub-millimeter resolution. Neuroimage 47(4):1244–1251. https://doi.org/10.1016/j.neuroimage.2009.05.098
Yano R, Hata J, Abe Y et al (2018) Quantitative temporal changes in DTI values coupled with histological properties in cuprizone-induced demyelination and remyelination. Neurochem Int 2018(119):151–158. https://doi.org/10.1016/j.neuint.2017.10.004
Ugwu ID, Amico F, Carballedo A et al (2015) Childhood adversity, depression, age and gender effects on white matter microstructure: a DTI study. Brain Struct Funct 220(4):1997–2009. https://doi.org/10.1007/s00429-014-0769-x
Jayantee K, Soni N, Deepanshu D et al (2018) Evaluation of optic nerve functions in subacute combined degeneration using visual evoked potential and diffusion tensor imaging—a pilot study. BJR. https://doi.org/10.1259/bjr.20180086
Kronlage M, Schwehr V, Schwarz D et al (2018) Peripheral nerve diffusion tensor imaging (DTI): normal values and demographic determinants in a cohort of 60 healthy individuals. Eur Radiol 28(5):1801–1808. https://doi.org/10.1007/s00330-017-5134-z
Zhang Q‑J, Wang DZ-LB et al (2015) Diffusion tensor imaging of optic nerve and optic radiation in primary chronic angle-closure glaucoma using 3T magnetic resonance imaging. Int J Ophthalmol 8(05):975–979. https://doi.org/10.3980/j.issn.2222-3959.2015.05.22
Tournier JD, Mori S, Leemans A (2011) Diffusion tensor imaging and beyond. Magn Reson Med 65(6):1532–1556. https://doi.org/10.1002/mrm.22924
Tuch DS, Timothy G et al (2010) High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med 48(4):577–582. https://doi.org/10.1002/mrm.10268
Dimond D, Schuetze M, Smith RE et al (2019) Reduced white matter fiber density in autism spectrum disorder. Cereb Cortex 29(4):1778–1788. https://doi.org/10.1093/cercor/bhy348
Raffelt DA, Tournier J‑D et al (2017) Investigating white matter fibre density and morphology using fixel-based analysis. Neuroimage 2017(144):58–73. https://doi.org/10.1016/j.neuroimage.2016.09.029
Remika M, Raffelt D, Dhollander T et al (2018) Fibre-specific white matter reductions in Alzheimer’s disease and mild cognitive impairment. Brain : A J Neurol. https://doi.org/10.1093/brain/awx355
Egorova N, Dhollander T, Khlif MS et al (2020) Pervasive white matter fiber degeneration in ischemic stroke. Stroke. https://doi.org/10.1161/STROKEAHA.119.028143
Ercan E, Varma G, Mädler B et al (2018) Microstructural correlates of 3D steady-state inhomogeneous magnetization transfer (ihMT) in the human brain white matter assessed by myelin water imaging and diffusion tensor imaging. Magn Reson Med. https://doi.org/10.1002/mrm.27211
Hoffmann C, Weisz B, Lipitz S et al (2014) Regional apparent diffusion coefficient values in 3rd trimester fetal brain. Neuroradiology. https://doi.org/10.1007/s00234-014-1359-6
Wu C‑N, Duan S‑F, Mu X‑T et al (2019) Assessment of optic nerve and optic tract alterations in patients with orbital space-occupying lesions using probabilistic diffusion tractography. Int J Ophthalmol 12(08):1304–1310. https://doi.org/10.18240/ijo.2019.08.11
Zhong Y‑F, Tang Z‑H, Qiang J‑W et al (2017) Changes in DTI parameters in the optic tracts of macaque monkeys with monocular blindness. Neurosci Lett 2017:636. https://doi.org/10.1016/j.neulet.2016.11.030
Hui E, Cheung M, Chan K et al (2010) B‑value dependence of DTI quantitation and sensitivity in detecting neural tissue changes. Neuroimage 49(3):2366–2374. https://doi.org/10.1016/j.neuroimage.2009.10.022
Genc S, Tax CMW, Raven EP, Chamberland M, Parker GD, Jones DK (2020) Impact of b‑value on estimates of apparent fibre density. Hum Brain Mapp 41(10):2583–2595
Author information
Authors and Affiliations
Contributions
Availability of data
The datasets used or analyzed during the current study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
Corresponding author
Ethics declarations
Conflict of interest
Y. Wang was supported by the Shandong Province Medical and Health Science and Technology Development Project (2016SW0606) and Shandong University Science and Technology Development Program (J15LL05). Y. Shao, L. Li, W. Peng, and W. Lu declare that they have no competing interests.
All procedures performed in studies involving human participants or on human tissue were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the ethics committee of Shandong First Medical University in accordance with the 1964 Helsinki Declaration and the later amendments, with approval number 2022046. Informed consent was obtained from all individual participants included in the study.
The supplement containing this article is not sponsored by industry.
Additional information

Scan QR code & read article online
Rights and permissions
About this article
Cite this article
Shao, Y., Li, L., Peng, W. et al. Age-related changes in the healthy adult visual pathway: evidence from diffusion tensor imaging with fixel-based analysis. Radiologie 63 (Suppl 2), 73–81 (2023). https://doi.org/10.1007/s00117-023-01192-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00117-023-01192-x