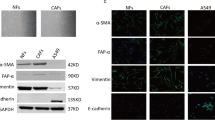
Abstract
Cancer-associated fibroblasts (CAFs) are important components in the tumor microenvironment, and we sought to identify effective therapeutic targets in CAFs for non-small cell lung cancer (NSCLC). In this study, we established fibroblast cell lines from the cancerous and non-cancerous parts of surgical lung specimens from patients with NSCLC and evaluated the differences in behaviors towards NSCLC cells. RNA sequencing analysis was performed to investigate the differentially expressed genes between normal fibroblasts (NFs) and CAFs, and we identified that the expression of periostin (POSTN), which is known to be overexpressed in various solid tumors and promote cancer progression, was significantly higher in CAFs than in NFs. POSTN increased cell proliferation via NSCLC cells’ ERK pathway activation and induced epithelial-mesenchymal transition (EMT), which improved migration in vitro. In addition, POSTN knockdown in CAFs suppressed these effects, and in vivo experiments demonstrated that the POSTN knockdown improved the sensitivity of EGFR-mutant NSCLC cells for osimertinib treatment. Collectively, our results showed that CAF-derived POSTN is involved in tumor growth, migration, EMT induction, and drug resistance in NSCLC. Targeting CAF-secreted POSTN could be a potential therapeutic strategy for NSCLC.
Key messages
• POSTN is significantly upregulated in CAFs compared to normal fibroblasts in NCSLC.
• POSTN increases cell proliferation via activation of the NSCLC cells’ ERK pathway.
• POSTN induces EMT in NSCLC cells and improves the migration ability.
• POSTN knockdown improves the sensitivity for osimertinib in EGFR-mutant NSCLC cells.




Similar content being viewed by others
Data availability
The raw RNA-seq data generated in this study are publicly available in Gene Expression Omnibus (GEO) at GSE205814. Data sources and handling of publicly available data are described in the Materials and Methods. Further information is available from the corresponding author upon request.
Change history
18 December 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00109-023-02408-2
Abbreviations
- αSMA:
-
Alpha-smooth muscle actin
- CAF:
-
Cancer-associated fibroblasts
- FAP:
-
Fibroblast activation protein
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- EMT:
-
Epithelial-mesenchymal transition
- MAPK:
-
Mitogen-activated protein kinase
- NF:
-
Normal fibroblasts
- NSCLC:
-
Non-small cell lung cancer
- POSTN:
-
Periostin
- TME:
-
Tumor microenvironment
References
Alexander M, Kim SY, Cheng H (2020) Update 2020: Management of Non-Small Cell Lung Cancer. Lung 198:897–907. https://doi.org/10.1007/s00408-020-00407-5
Quail DF, Joyce JA (2013) Microenvironmental regulation of tumor progression and metastasis. Nat Med 19:1423–1437. https://doi.org/10.1038/nm.3394
Fiori ME, Di Franco S, Villanova L, Bianca P, Stassi G, De Maria R (2019) Cancer-associated fibroblasts as abettors of tumor progression at the crossroads of EMT and therapy resistance. Mol Cancer 18:70. https://doi.org/10.1186/s12943-019-0994-2
Sun W, Fu S (2019) Role of cancer-associated fibroblasts in tumor structure, composition and the microenvironment in ovarian cancer. Oncol Lett 18:2173–2178. https://doi.org/10.3892/ol.2019.10587
Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A (1999) Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res 14:1239–1249. https://doi.org/10.1359/jbmr.1999.14.7.1239
Cui D, Huang Z, Liu Y, Ouyang G (2017) The multifaceted role of periostin in priming the tumor microenvironments for tumor progression. Cell Mol Life Sci 74:4287–4291. https://doi.org/10.1007/s00018-017-2646-2
González-González L, Alonso J (2018) Periostin: A Matricellular Protein With Multiple Functions in Cancer Development and Progression. Front Oncol 8:225. https://doi.org/10.3389/fonc.2018.00225
Rachner TD, Göbel A, Hoffmann O, Erdmann K, Kasimir-Bauer S, Breining D, Kimmig R, Hofbauer LC, Bittner AK (2020) High serum levels of periostin are associated with a poor survival in breast cancer. Breast Cancer Res Treat 180:515–524. https://doi.org/10.1007/s10549-020-05570-0
Dong D, Zhang L, Jia L, Ji W, Wang Z, Ren L, Niu R, Zhou Y (2018) Identification of Serum Periostin as a Potential Diagnostic and Prognostic Marker for Colorectal Cancer. Clin Lab 64:973–981. https://doi.org/10.7754/Clin.Lab.2018.171225
Lv Y, Wang W, Jia WD, Sun QK, Huang M, Zhou HC, Xia HH, Liu WB, Chen H, Sun SN et al (2013) High preoparative levels of serum periostin are associated with poor prognosis in patients with hepatocellular carcinoma after hepatectomy. Eur J Surg Oncol 39:1129–1135. https://doi.org/10.1016/j.ejso.2013.06.023
Xu CH, Wang W, Lin Y, Qian LH, Zhang XW, Wang QB, Yu LK (2017) Diagnostic and prognostic value of serum periostin in patients with non-small cell lung cancer. Oncotarget 8:18746–18753. https://doi.org/10.18632/oncotarget.13004
Qin X, Yan M, Zhang J, Wang X, Shen Z, Lv Z, Li Z, Wei W, Chen W (2016) TGFβ3-mediated induction of Periostin facilitates head and neck cancer growth and is associated with metastasis. Sci Rep 6:20587. https://doi.org/10.1038/srep20587
Ochi K, Suzawa K, Thu YM, Takatsu F, Tsudaka S, Zhu Y, Nakata K, Takeda T, Shien K, Yamamoto H et al (2022) Drug repositioning of tranilast to sensitize a cancer therapy by targeting cancer-associated fibroblast. Cancer Sci. https://doi.org/10.1111/cas.15502
Kim N, Kim HK, Lee K, Hong Y, Cho JH, Choi JW, Lee JI, Suh YL, Ku BM, Eum HH et al (2020) Single-cell RNA sequencing demonstrates the molecular and cellular reprogramming of metastatic lung adenocarcinoma. Nat Commun 11:2285. https://doi.org/10.1038/s41467-020-16164-1
Bartha Á, Győrffy B (2021) TNMplot.com: A Web Tool for the Comparison of Gene Expression in Normal, Tumor and Metastatic Tissues. Int J Mol Sci 22. https://doi.org/10.3390/ijms22052622
Chen X, Song E (2019) Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov 18:99–115. https://doi.org/10.1038/s41573-018-0004-1
Ruan K, Bao S, Ouyang G (2009) The multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci 66:2219–2230. https://doi.org/10.1007/s00018-009-0013-7
Roma-Rodrigues C, Mendes R, Baptista PV, Fernandes AR (2019) Targeting Tumor Microenvironment for Cancer Therapy. Int J Mol Sci 20. https://doi.org/10.3390/ijms20040840
Kalluri R, Zeisberg M (2006) Fibroblasts in cancer. Nat Rev Cancer 6:392–401. https://doi.org/10.1038/nrc1877
Kanzaki R, Pietras K (2020) Heterogeneity of cancer-associated fibroblasts: Opportunities for precision medicine. Cancer Sci 111:2708–2717. https://doi.org/10.1111/cas.14537
Nitsche U, Stangel D, Pan Z, Schlitter AM, Esposito I, Regel I, Raulefs S, Friess H, Kleeff J, Erkan M (2016) Periostin and tumor-stroma interactions in non-small cell lung cancer. Oncol Lett 12:3804–3810. https://doi.org/10.3892/ol.2016.5132
Hong LZ, Wei XW, Chen JF, Shi Y (2013) Overexpression of periostin predicts poor prognosis in non-small cell lung cancer. Oncol Lett 6:1595–1603. https://doi.org/10.3892/ol.2013.1590
Ratajczak-Wielgomas K, Kmiecik A, Grzegrzołka J, Piotrowska A, Gomulkiewicz A, Partynska A, Pawelczyk K, Nowinska K, Podhorska-Okolow M, Dziegiel P (2020) Prognostic Significance of Stromal Periostin Expression in Non-Small Cell Lung Cancer. Int J Mol Sci 21. https://doi.org/10.3390/ijms21197025
Zhang Y, Yuan D, Yao Y, Sun W, Shi Y, Su X (2017) Predictive and prognostic value of serum periostin in advanced non-small cell lung cancer patients receiving chemotherapy. Tumour Biol 39:1010428317698367. https://doi.org/10.1177/1010428317698367
Che J, Shen WZ, Deng Y, Dai YH, Liao YD, Yuan XL, Zhang P (2017) Effects of lentivirus-mediated silencing of Periostin on tumor microenvironment and bone metastasis via the integrin-signaling pathway in lung cancer. Life Sci 182:10–21. https://doi.org/10.1016/j.lfs.2017.05.030
Berdiel-Acer M, Sanz-Pamplona R, Calon A, Cuadras D, Berenguer A, Sanjuan X, Paules MJ, Salazar R, Moreno V, Batlle E et al (2014) Differences between CAFs and their paired NCF from adjacent colonic mucosa reveal functional heterogeneity of CAFs, providing prognostic information. Mol Oncol 8:1290–1305. https://doi.org/10.1016/j.molonc.2014.04.006
Liu Y, Huang Z, Cui D, Ouyang G (2019) The Multiaspect Functions of Periostin in Tumor Progression. Adv Exp Med Biol 1132:125–136. https://doi.org/10.1007/978-981-13-6657-4_13
Du B, Shim JS (2016) Targeting Epithelial-Mesenchymal Transition (EMT) to Overcome Drug Resistance in Cancer. Molecules 21. https://doi.org/10.3390/molecules21070965
Okazaki T, Tamai K, Shibuya R, Nakamura M, Mochizuki M, Yamaguchi K, Abe J, Takahashi S, Sato I, Kudo A et al (2018) Periostin is a negative prognostic factor and promotes cancer cell proliferation in non-small cell lung cancer. Oncotarget 9:31187–31199. https://doi.org/10.18632/oncotarget.25435
Hu WW, Chen PC, Chen JM, Wu YM, Liu PY, Lu CH, Lin YF, Tang CH, Chao CC (2017) Periostin promotes epithelial-mesenchymal transition via the MAPK/miR-381 axis in lung cancer. Oncotarget 8:62248–62260. https://doi.org/10.18632/oncotarget.19273
Wu SG, Shih JY (2018) Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol Cancer 17:38. https://doi.org/10.1186/s12943-018-0777-1
Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, Orme JP, Finlay MR, Ward RA, Mellor MJ et al (2014) AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 4:1046–1061. https://doi.org/10.1158/2159-8290.Cd-14-0337
Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M (2019) Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer 121:725–737. https://doi.org/10.1038/s41416-019-0573-8
Tricker EM, Xu C, Uddin S, Capelletti M, Ercan D, Ogino A, Pratilas CA, Rosen N, Gray NS, Wong KK et al (2015) Combined EGFR/MEK Inhibition Prevents the Emergence of Resistance in EGFR-Mutant Lung Cancer. Cancer Discov 5:960–971. https://doi.org/10.1158/2159-8290.Cd-15-0063
Biffi G, Tuveson DA (2021) Diversity and Biology of Cancer-Associated Fibroblasts. Physiol Rev 101:147–176. https://doi.org/10.1152/physrev.00048.2019
Kalluri R (2016) The biology and function of fibroblasts in cancer. Nat Rev Cancer 16:582–598. https://doi.org/10.1038/nrc.2016.73
Acknowledgements
The authors thank Ms. Fumiko Isobe for her technical assistance.
Funding
This study was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (Grant number 22529148).
Author information
Authors and Affiliations
Contributions
Conception and design: F. Takatsu, K. Suzawa, S. Tomida, M. Sakaguchi, and S. Toyooka. Acquisition of data: F. Takatsu, S. Tomida, and Y. Thu. Analysis and interpretation of data: F. Takatsu, K. Suzawa, S. Tomida, M. Sakaguchi, T. Toji, M. Ohki, S. Tsudaka, K. Date, N. Matsuda, K. Iwata, Y. Zhu, K. Nakata, K. Shien, H. Yamamoto, A. Nakayama, M. Okazaki, S. Sugimoto, and S. Toyooka. Writing, review, and revision of the manuscript: F. Takatsu, K. Suzawa, S. Tomida, and S. Toyooka. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Studies using clinical specimens were approved by the Okayama Medical School and Hospital’s Research Ethics Committee (Approval Number: # 1906–033). The protocol for animal models was approved by the Animal Care and Use Committee of Okayama University (Okayama, Japan: permit number: OKU-2016398), and this study was carried out in accordance with the Guidelines of the Okayama University.
Consent to participate
Informed consent was obtained from all individual patients for the use of their materials.
Competing interests
Shinichi Toyooka received research funding from Eli Lilly Japan, Taiho (Japan) and Chugai (Japan), and lecture fees from Chugai. All other authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Takatsu, F., Suzawa, K., Tomida, S. et al. Periostin secreted by cancer-associated fibroblasts promotes cancer progression and drug resistance in non-small cell lung cancer. J Mol Med 101, 1603–1614 (2023). https://doi.org/10.1007/s00109-023-02384-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-023-02384-7