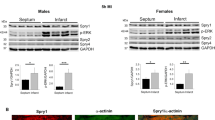
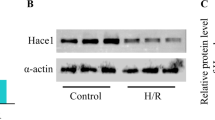
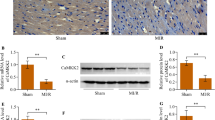
Abstract
Reperfusion after acute myocardial infarction further exaggerates cardiac injury and adverse remodeling. Irrespective of cardiac cell types, loss of specifically the α isoform of the protein kinase GSK-3 is protective in chronic cardiac diseases. However, the role of GSK-3α in clinically relevant ischemia/reperfusion (I/R)-induced cardiac injury is unknown. Here, we challenged cardiomyocyte-specific conditional GSK-3α knockout (cKO) and littermate control mice with I/R injury and investigated the underlying molecular mechanism using an in vitro GSK-3α gain-of-function model in AC16 cardiomyocytes post-hypoxia/reoxygenation (H/R). Analysis revealed a significantly lower percentage of infarct area in the cKO vs. control hearts post-I/R. Consistent with in vivo findings, GSK-3α overexpression promoted AC16 cardiomyocyte death post-H/R which was accompanied by an induction of reactive oxygen species (ROS) generation. Consistently, GSK-3α gain-of-function caused mitochondrial dysfunction by significantly suppressing mitochondrial membrane potential. Transcriptomic analysis of GSK-3α overexpressing cardiomyocytes challenged with hypoxia or H/R revealed that NOD-like receptor (NLR), TNF, NF-κB, IL-17, and mitogen-activated protein kinase (MAPK) signaling pathways were among the most upregulated pathways. Glutathione and fatty acid metabolism were among the top downregulated pathways post-H/R. Together, these observations suggest that loss of cardiomyocyte-GSK-3α attenuates cardiac injury post-I/R potentially through limiting the myocardial inflammation, mitochondrial dysfunction, and metabolic derangement. Therefore, selective inhibition of GSK-3α may provide beneficial effects in I/R-induced cardiac injury and remodeling.
Key messages
-
GSK-3α promotes cardiac injury post-ischemia/reperfusion (I/R).
-
GSK-3α regulates inflammatory and metabolic pathways post-hypoxia/reoxygenation (H/R).
-
GSK-3α overexpression upregulates NOD-like receptor (NLR), TNF, NF-kB, IL-17, and MAPK signaling pathways in cardiomyocytes post-H/R.
-
GSK-3α downregulates glutathione and fatty acid metabolic pathways in cardiomyocytes post-H/R.








Similar content being viewed by others
Data availability
Data is included in the manuscript and supplementary files. All the raw data is deposited in the NCBI database with the accession number PRJNA984601.
References
Whelan RS, Kaplinskiy V, Kitsis RN (2010) Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol 72:19–44. https://doi.org/10.1146/annurev.physiol.010908.163111
Cormier KW, Woodgett JR (2017) Recent advances in understanding the cellular roles of GSK-3. F1000Res 6. https://doi.org/10.12688/f1000research.10557.1
Lal H, Ahmad F, Woodgett J, Force T (2015) The GSK-3 family as therapeutic target for myocardial diseases. Circ Res 116:138–149. https://doi.org/10.1161/CIRCRESAHA.116.303613
Ahmad F, Woodgett JR (2020) Emerging roles of GSK-3alpha in pathophysiology: emphasis on cardio-metabolic disorders. Biochim Biophys Acta Mol Cell Res 1867:118616. https://doi.org/10.1016/j.bbamcr.2019.118616
Gupte M, Tumuluru S, Sui JY, Singh AP, Umbarkar P, Parikh SS, Ahmad F, Zhang Q, Force T, Lal H (2018) Cardiomyocyte-specific deletion of GSK-3beta leads to cardiac dysfunction in a diet induced obesity model. Int J Cardiol 259:145–152. https://doi.org/10.1016/j.ijcard.2018.01.013
Zhang N, Tian YN, Zhou LN, Li MZ, Chen HD, Song SS, Huan XJ, Bao XB, Zhang A, Miao ZH et al (2021) Glycogen synthase kinase 3beta inhibition synergizes with PARP inhibitors through the induction of homologous recombination deficiency in colorectal cancer. Cell Death Dis 12:183. https://doi.org/10.1038/s41419-021-03475-4
Griebel G, Stemmelin J, Lopez-Grancha M, Boulay D, Boquet G, Slowinski F, Pichat P, Beeske S, Tanaka S, Mori A et al (2019) The selective GSK3 inhibitor, SAR502250, displays neuroprotective activity and attenuates behavioral impairments in models of neuropsychiatric symptoms of Alzheimer’s disease in rodents. Sci Rep 9:18045. https://doi.org/10.1038/s41598-019-54557-5
Kerkela R, Kockeritz L, Macaulay K, Zhou J, Doble BW, Beahm C, Greytak S, Woulfe K, Trivedi CM, Woodgett JR et al (2008) Deletion of GSK-3beta in mice leads to hypertrophic cardiomyopathy secondary to cardiomyoblast hyperproliferation. J Clin Invest 118:3609–3618
Zhou J, Freeman TA, Ahmad F, Shang X, Mangano E, Gao E, Farber J, Wang Y, Ma XL, Woodgett J et al (2013) GSK-3alpha is a central regulator of age-related pathologies in mice. J Clin Invest 123: 1821–1832. https://doi.org/10.1172/JCI64398
Lal H, Zhou J, Ahmad F, Zaka R, Vagnozzi RJ, Decaul M, Woodgett J, Gao E, Force T (2012) Glycogen synthase kinase-3alpha limits ischemic injury, cardiac rupture, post-myocardial infarction remodeling and death. Circulation 125:65–75. https://doi.org/10.1161/CIRCULATIONAHA.111.050666
Embi N, Rylatt DB, Cohen P (1980) Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem 107:519–527
Woodgett JR (1991) cDNA cloning and properties of glycogen synthase kinase-3. Methods Enzymol 200:564–577
MacAulay K, Doble BW, Patel S, Hansotia T, Sinclair EM, Drucker DJ, Nagy A, Woodgett JR (2007) Glycogen synthase kinase 3alpha-specific regulation of murine hepatic glycogen metabolism. Cell Metab 6:329–337. https://doi.org/10.1016/j.cmet.2007.08.013
Cohen P (2002) Protein kinases- the major drug targets of the twenty-first century? Nat Rev Drug Disc 1:309–315
Linkermann A, Brasen JH, Darding M, Jin MK, Sanz AB, Heller JO, De Zen F, Weinlich R, Ortiz A, Walczak H et al (2013) Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci USA 110:12024–12029. https://doi.org/10.1073/pnas.1305538110
Eltzschig HK, Eckle T (2011) Ischemia and reperfusion–from mechanism to translation. Nat Med 17:1391–1401. https://doi.org/10.1038/nm.2507
Matsuda T, Zhai P, Maejima Y, Hong C, Gao S, Tian B, Goto K, Takagi H, Tamamori-Adachi M, Kitajima S et al (2008) Distinct roles of GSK-3alpha and GSK-3beta phosphorylation in the heart under pressure overload. Proc Natl Acad Sci USA 105:20900–20905
Ahmad F, Lal H, Zhou J, Vagnozzi RJ, Yu JE, Shang X, Woodgett JR, Gao E, Force T (2014) Cardiomyocyte-specific deletion of gsk3alpha mitigates post-myocardial infarction remodeling, contractile dysfunction, and heart failure. J Am Coll Cardiol 64:696–706. https://doi.org/10.1016/j.jacc.2014.04.068
Umbarkar P, Tousif S, Singh AP, Anderson JC, Zhang Q, Tallquist MD, Woodgett J, Lal H (2022) Fibroblast GSK-3alpha promotes fibrosis via RAF-MEK-ERK pathway in the injured heart. Circ Res 131:620–636. https://doi.org/10.1161/CIRCRESAHA.122.321431
Ahmad F, Singh AP, Tomar D, Rahmani M, Zhang Q, Woodgett JR, Tilley DG, Lal H, Force T (2019) Cardiomyocyte-GSK-3alpha promotes mPTP opening and heart failure in mice with chronic pressure overload. J Mol Cell Cardiol 130:65–75. https://doi.org/10.1016/j.yjmcc.2019.03.020
Woulfe KC, Gao E, Lal H, Harris D, Fan Q, Vagnozzi R, DeCaul M, Shang X, Patel S, Woodgett JR et al (2010) Glycogen synthase kinase-3beta regulates post-myocardial infarction remodeling and stress-induced cardiomyocyte proliferation in vivo. Circ Res 106:1635–1645. https://doi.org/10.1161/CIRCRESAHA.109.211482
Lal H, Ahmad F, Zhou J, Yu JE, Vagnozzi RJ, Guo Y, Yu D, Tsai EJ, Woodgett J, Gao E et al (2014) Cardiac fibroblast glycogen synthase kinase-3beta regulates ventricular remodeling and dysfunction in ischemic heart. Circulation 130:419–430. https://doi.org/10.1161/CIRCULATIONAHA.113.008364
Zhou J, Ahmad F, Parikh S, Hoffman NE, Rajan S, Verma VK, Song J, Yuan A, Shanmughapriya S, Guo Y et al (2016) Loss of adult cardiac myocyte GSK-3 leads to mitotic catastrophe resulting in fatal dilated cardiomyopathy. Circ Res 118:1208–1222. https://doi.org/10.1161/CIRCRESAHA.116.308544
Zhou J, Ahmad F, Lal H, Force T (2016) Response by Zhou et al to letter regarding article, “Loss of adult cardiac myocyte GSK-3 leads to mitotic catastrophe resulting in fatal dilated cardiomyopathy.” Circ Res 119:e29–e30. https://doi.org/10.1161/CIRCRESAHA.116.309093
Gao E, Lei YH, Shang X, Huang ZM, Zuo L, Boucher M, Fan Q, Chuprun JK, Ma XL, Koch WJ (2010) A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circ Res 107:1445–1453
Yusuf AM, Qaisar R, Al-Tamimi AO, Jayakumar MN, Woodgett JR, Koch WJ, Ahmad F (2022) Cardiomyocyte-GSK-3beta deficiency induces cardiac progenitor cell proliferation in the ischemic heart through paracrine mechanisms. J Cell Physiol 237:1804–1817. https://doi.org/10.1002/jcp.30644
Ahmad F, Tomar D, Aryal ACS, Elmoselhi AB, Thomas M, Elrod JW, Tilley DG, Force T (2020) Nicotinamide riboside kinase-2 alleviates ischemia-induced heart failure through P38 signaling. Biochim Biophys Acta Mol Basis Dis 1866:165609. https://doi.org/10.1016/j.bbadis.2019.165609
Shahzadi SK, Marzook H, Qaisar R, Ahmad F (2022) Nicotinamide riboside kinase-2 inhibits JNK pathway and limits dilated cardiomyopathy in mice with chronic pressure overload. Clin Sci (Lond) 136:181–196. https://doi.org/10.1042/CS20210964
Marzook H, Gupta A, Tomar D, Saleh MA, Patil K, Semreen MH, Hamoudi R, Soares NC, Qaisar R, Ahmad F (2023) Nicotinamide riboside kinase-2 regulates metabolic adaptation in the ischemic heart. J Mol Med (Berl) 101:311–326. https://doi.org/10.1007/s00109-023-02296-6
Khan AA, Gul MT, Karim A, Ranade A, Azeem M, Ibrahim Z, Ramachandran G, Nair VA, Ahmad F, Elmoselhi A et al (2022) Mitigating sarcoplasmic reticulum stress limits disuse-induced muscle loss in hindlimb unloaded mice. NPJ Microgravity 8:24. https://doi.org/10.1038/s41526-022-00211-w
Gupte M, Lal H, Ahmad F, Sawyer DB, Hill MF (2017) Chronic neuregulin-1beta treatment mitigates the progression of postmyocardial infarction heart failure in the setting of type 1 diabetes mellitus by suppressing myocardial apoptosis, Fibrosis, and Key Oxidant-Producing Enzymes. J Card Fail 23:887–899. https://doi.org/10.1016/j.cardfail.2017.08.456
Zorova LD, Popkov VA, Plotnikov EY, Silachev DN, Pevzner IB, Jankauskas SS, Babenko VA, Zorov SD, Balakireva AV, Juhaszova M et al (2018) Mitochondrial membrane potential. Anal Biochem 552:50–59. https://doi.org/10.1016/j.ab.2017.07.009
Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard MH, Force T, Franke T, Hajjar R, Rosenzweig A (2001) Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation 104:330–335
Ghafouri-Fard S, Khanbabapour Sasi A, Hussen BM, Shoorei H, Siddiq A, Taheri M, Ayatollahi SA (2022) Interplay between PI3K/AKT pathway and heart disorders. Mol Biol Rep 49:9767–9781. https://doi.org/10.1007/s11033-022-07468-0
Ruderman NB, Kapeller R, White MF, Cantley LC (1990) Activation of phosphatidylinositol 3-kinase by insulin. Proc Natl Acad Sci USA 87:1411–1415. https://doi.org/10.1073/pnas.87.4.1411
Zhai P, Sciarretta S, Galeotti J, Volpe M, Sadoshima J (2011) Differential roles of GSK-3beta during myocardial ischemia and ischemia/reperfusion. Circ Res 109:502–511. https://doi.org/10.1161/CIRCRESAHA.111.249532
Cadenas S (2018) ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic Biol Med 117:76–89. https://doi.org/10.1016/j.freeradbiomed.2018.01.024
Sharma HS, Das DK (1997) Role of cytokines in myocardial ischemia and reperfusion. Mediators Inflamm 6:175–183. https://doi.org/10.1080/09629359791668
Schutte-Nutgen K, Edeling M, Kentrup D, Heitplatz B, Van Marck V, Zarbock A, Meersch-Dini M, Pavenstadt H, Reuter S (2022) Interleukin 24 promotes cell death in renal epithelial cells and is associated with acute renal injury. Am J Transplant 22:2548–2559. https://doi.org/10.1111/ajt.17143
Buzas K, Oppenheim JJ, Zack Howard OM (2011) Myeloid cells migrate in response to IL-24. Cytokine 55:429–434. https://doi.org/10.1016/j.cyto.2011.05.018
Menezes ME, Bhoopathi P, Pradhan AK, Emdad L, Das SK, Guo C, Wang XY, Sarkar D, Fisher PB (2018) Role of MDA-7/IL-24 a Multifunction protein in human diseases. Adv Cancer Res 138:143–182. https://doi.org/10.1016/bs.acr.2018.02.005
Hartupee J, Liu C, Novotny M, Li X, Hamilton T (2007) IL-17 enhances chemokine gene expression through mRNA stabilization. J Immunol 179:4135–4141. https://doi.org/10.4049/jimmunol.179.6.4135
Xu S, Cao X (2010) Interleukin-17 and its expanding biological functions. Cell Mol Immunol 7:164–174. https://doi.org/10.1038/cmi.2010.21
Xiao L, Zhang WH, Huang Y, Huang P (2022) Intestinal ischemia-reperfusion induces the release of IL-17A to regulate cell inflammation, apoptosis and barrier damage. Exp Ther Med 23:158. https://doi.org/10.3892/etm.2021.11081
Zhang J, Li Q, Zou YR, Wu SK, Lu XH, Li GS, Wang J (2021) HMGB1-TLR4-IL-23-IL-17A axis accelerates renal ischemia-reperfusion injury via the recruitment and migration of neutrophils. Int Immunopharmacol 94:107433. https://doi.org/10.1016/j.intimp.2021.107433
Matuz-Mares D, Riveros-Rosas H, Vilchis-Landeros MM, Vazquez-Meza H (2021) Glutathione participation in the prevention of cardiovascular diseases. Antioxidants (Basel) 10. https://doi.org/10.3390/antiox10081220
Tan M, Yin Y, Ma X, Zhang J, Pan W, Tan M, Zhao Y, Yang T, Jiang T, Li H (2023) Glutathione system enhancement for cardiac protection: pharmacological options against oxidative stress and ferroptosis. Cell Death Dis 14:131. https://doi.org/10.1038/s41419-023-05645-y
Fan Q, Yang XC, Cao XB, Wang SY, Yang SL, Liu XL, Gao F (2006) Glutathione reverses peroxynitrite-mediated deleterious effects of nitroglycerin on ischemic rat hearts. J Cardiovasc Pharmacol 47:405–412. https://doi.org/10.1097/01.fjc.0000210073.48991.bf
Cheung PY, Wang W, Schulz R (2000) Glutathione protects against myocardial ischemia-reperfusion injury by detoxifying peroxynitrite. J Mol Cell Cardiol 32:1669–1678. https://doi.org/10.1006/jmcc.2000.1203
Funding
The work was supported by collaborative (22010901112) research grants from the University of Sharjah to Firdos Ahmad.
Author information
Authors and Affiliations
Contributions
FA designed the study, performed experiments, acquired funding, supervised the project, performed data analysis and interpretation, and wrote the manuscript. HM performed experiments, collected data, and helped with manuscript writing. AA and AG performed experiments and collected data. AAK and MAS helped with data analysis, interpretation, and manuscript writing. WJK and JRW helped with reagents, data analysis, and manuscript editing. RQ helped with data analysis, interpretation, and supervision.
Corresponding author
Ethics declarations
Ethics approval
The Institutional Animal Care and Use Committee (IACUC) of Vanderbilt University Medical Center approved all animal procedures and treatments.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmad, F., Marzook, H., Gupta, A. et al. GSK-3α aggravates inflammation, metabolic derangement, and cardiac injury post-ischemia/reperfusion. J Mol Med 101, 1379–1396 (2023). https://doi.org/10.1007/s00109-023-02373-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-023-02373-w