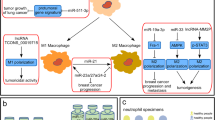
Abstract
Gastric cancer remains one of the cancers with the highest mortality in the world; therefore, it is very important to investigate its pathogenesis to improve the prognosis of gastric cancer patients. Recently, noncoding RNAs have become a research hotspot in the field of oncology. These RNA molecules play complex roles in the regulation of tumor cells, immune cells, and the tumor microenvironment. Therefore, studying their ability to regulate the gastric cancer immune microenvironment will provide us with a better perspective to understand their potential role in anticancer therapy. In this review, we discuss the regulatory effects of several common noncoding RNAs on the immune microenvironment of gastric cancer and their prospects in anticancer therapy to provide some novel insight into the identification of valuable diagnostic markers and improving the prognosis of gastric cancer patients.

Similar content being viewed by others
References
Joshi SS, Badgwell BD (2021) Current treatment and recent progress in gastric cancer. CA Cancer J Clin 71:264–279. https://doi.org/10.3322/caac.21657
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Necula L, Matei L, Dragu D, Neagu AI, Mambet C, Nedeianu S, Bleotu C, Diaconu CC, Chivu-Economescu M (2019) Recent advances in gastric cancer early diagnosis. World J Gastroenterol 25:2029–2044. https://doi.org/10.3748/wjg.v25.i17.2029
Nagini S (2012) Carcinoma of the stomach: a review of epidemiology, pathogenesis, molecular genetics and chemoprevention. World J Gastrointest Oncol 4:156–169. https://doi.org/10.4251/wjgo.v4.i7.156
Fattahi S, Kosari-Monfared M, Ghadami E, Golpour M, Khodadadi P, Ghasemiyan M, Akhavan-Niaki H (2018) Infection-associated epigenetic alterations in gastric cancer: new insight in cancer therapy. J Cell Physiol 233:9261–9270. https://doi.org/10.1002/jcp.27030
Puneet Kazmi HR, Kumari S, Tiwari S, Khanna A, Narayan G (2018) Epigenetic mechanisms and events in gastric cancer-emerging novel biomarkers. Pathol Oncol Res 24:757–770. https://doi.org/10.1007/s12253-018-0410-z
Sonohara F, Inokawa Y, Hayashi M, Kodera Y, Nomoto S (2017) Epigenetic modulation associated with carcinogenesis and prognosis of human gastric cancer. Oncol Lett 13:3363–3368. https://doi.org/10.3892/ol.2017.5912
Fabbri M, Girnita L, Varani G, Calin GA (2019) Decrypting noncoding RNA interactions, structures, and functional networks. Genome Res 29:1377–1388. https://doi.org/10.1101/gr.247239.118
Slack FJ, Chinnaiyan AM (2019) The role of non-coding RNAs in oncology. Cell 179:1033–1055. https://doi.org/10.1016/j.cell.2019.10.017
Bure I, Haller F, Zaletaev DV (2018) Coding and non-coding: molecular portrait of GIST and its clinical implication. Curr Mol Med 18:252–259. https://doi.org/10.2174/1566524018666181004113436
Lee H, Zhang Z, Krause HM (2019) Long noncoding RNAs and repetitive elements: junk or intimate evolutionary partners? Trends Genet 35:892–902. https://doi.org/10.1016/j.tig.2019.09.006
Yaman Agaoglu F, Kovancilar M, Dizdar Y, Darendeliler E, Holdenrieder S, Dalay N, Gezer U (2011) Investigation of miR-21, miR-141, and miR-221 in blood circulation of patients with prostate cancer. Tumour Biol 32:583–588. https://doi.org/10.1007/s13277-011-0154-9
Fabris L, Ceder Y, Chinnaiyan AM, Jenster GW, Sorensen KD, Tomlins S, Visakorpi T, Calin GA (2016) The potential of MicroRNAs as prostate cancer biomarkers. Eur Urol 70:312–322. https://doi.org/10.1016/j.eururo.2015.12.054
Zhang X, Xie K, Zhou H, Wu Y, Li C, Liu Y, Liu Z, Xu Q, Liu S, Xiao D et al (2020) Role of non-coding RNAs and RNA modifiers in cancer therapy resistance. Mol Cancer 19:47. https://doi.org/10.1186/s12943-020-01171-z
Zheng A, Song X, Zhang L, Zhao L, Mao X, Wei M, Jin F (2019) Long non-coding RNA LUCAT1/miR-5582-3p/TCF7L2 axis regulates breast cancer stemness via Wnt/β-catenin pathway. J Exp Clin Cancer Res 38:305. https://doi.org/10.1186/s13046-019-1315-8
Salati M, Braconi C (2019) Noncoding RNA in cholangiocarcinoma. Semin Liver Dis 39:13–25. https://doi.org/10.1055/s-0038-1676097
Okugawa Y, Grady WM, Goel A (2015) Epigenetic alterations in colorectal cancer: emerging biomarkers. Gastroenterology. https://doi.org/10.1053/j.gastro.2015.07.011
Panni S, Lovering RC, Porras P, Orchard S (2020) Non-coding RNA regulatory networks. Biochim Biophys Acta Gene Regul Mech 1863. https://doi.org/10.1016/j.bbagrm.2019.194417
Arif KMT, Elliott EK, Haupt LM, Griffiths LR (2020) Regulatory mechanisms of epigenetic mirna relationships in human cancer and potential as therapeutic targets. Cancers (Basel). https://doi.org/10.3390/cancers12102922
Kalluri R, Zeisberg M (2006) Fibroblasts in cancer. Nat Rev Cancer 6:392–401. https://doi.org/10.1038/nrc1877
Kobayashi H, Enomoto A, Woods SL, Burt AD, Takahashi M, Worthley DL (2019) Cancer-associated fibroblasts in gastrointestinal cancer. Nat Rev Gastroenterol Hepatol 16:282–295. https://doi.org/10.1038/s41575-019-0115-0
Kalluri R (2016) The biology and function of fibroblasts in cancer. Nat Rev Cancer 16:582–598. https://doi.org/10.1038/nrc.2016.73
Zhao J, Du P, Cui P, Qin Y, Hu Ce WuJ, Zhou Z, Zhang W, Qin L, Huang G (2018) LncRNA PVT1 promotes angiogenesis via activating the STAT3/VEGFA axis in gastric cancer. Oncogene 37:4094–4109. https://doi.org/10.1038/s41388-018-0250-z
Li S, Liang X, Ma L, Shen L, Li T, Zheng L, Sun A, Shang W, Chen C, Zhao W et al (2018) MiR-22 sustains NLRP3 expression and attenuates H. pylori-induced gastric carcinogenesis. Oncogene 37:884–896. https://doi.org/10.1038/onc.2017.381
Liu R, Lu Z, Gu J, Liu J, Huang E, Liu X, Wang L, Yang J, Deng Y, Qian J et al (2018) MicroRNAs 15A and 16–1 activate signaling pathways that mediate chemotaxis of immune regulatory B cells to colorectal tumors. Gastroenterology 154(637–651). https://doi.org/10.1053/j.gastro.2017.09.045
Kopetz S, Grothey A, Yaeger R, Van Cutsem E, Desai J, Yoshino T, Wasan H, Ciardiello F, Loupakis F, Hong YS et al (2019) Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N Engl J Med 381:1632–1643. https://doi.org/10.1056/NEJMoa1908075
Ajani JA, Lee J, Sano T, Janjigian YY, Fan D, Song S (2017) Gastric adenocarcinoma. Nat Rev Dis Primers 3:17036. https://doi.org/10.1038/nrdp.2017.36
Mactier KE, Glaire MA, Basavaraju U, El-Omar EM, Hold GL (2014) MicroRNAs in gastrointestinal malignancy: a tool in cancer prevention? Eur J Cancer Prev 23:540–549. https://doi.org/10.1097/CEJ.0000000000000014
Tu C, Zeng Z, Qi P, Li X, Guo C, Xiong F, Xiang B, Zhou M, Liao Q, Yu J et al (2018) Identification of genomic alterations in nasopharyngeal carcinoma and nasopharyngeal carcinoma-derived Epstein-Barr virus by whole-genome sequencing. Carcinogenesis 39:1517–1528. https://doi.org/10.1093/carcin/bgy108
Yi M, Cai J, Li J, Chen S, Zeng Z, Peng Q, Ban Y, Zhou Y, Li X, Xiong W et al (2018) Rediscovery of NF-kappaB signaling in nasopharyngeal carcinoma: how genetic defects of NF-kappaB pathway interplay with EBV in driving oncogenesis? J Cell Physiol 233:5537–5549. https://doi.org/10.1002/jcp.26410
Oya Y, Hayakawa Y, Koike K (2020) Tumor microenvironment in gastric cancers. Cancer Sci 111:2696–2707. https://doi.org/10.1111/cas.14521
Huang T, Song C, Zheng L, Xia L, Li Y, Zhou Y (2019) The roles of extracellular vesicles in gastric cancer development, microenvironment, anti-cancer drug resistance, and therapy. Mol Cancer 18:62. https://doi.org/10.1186/s12943-019-0967-5
Fu M, Gu J, Jiang P, Qian H, Xu W, Zhang X (2019) Exosomes in gastric cancer: roles, mechanisms, and applications. Mol Cancer 18:41. https://doi.org/10.1186/s12943-019-1001-7
Aktas ON, Ozturk AB, Erman B, Erus S, Tanju S, Dilege S (2018) Role of natural killer cells in lung cancer. J Cancer Res Clin Oncol 144:997–1003. https://doi.org/10.1007/s00432-018-2635-3
Lu T, Ramakrishnan R, Altiok S, Youn JI, Cheng P, Celis E, Pisarev V, Sherman S, Sporn MB, Gabrilovich D (2011) Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J Clin Invest 121:4015–4029. https://doi.org/10.1172/JCI45862
Talmadge JE, Gabrilovich DI (2013) History of myeloid-derived suppressor cells. Nat Rev Cancer 13:739–752. https://doi.org/10.1038/nrc3581
Jackaman C, Tomay F, Duong L, Abdol Razak NB, Pixley FJ, Metharom P, Nelson DJ (2017) Aging and cancer: the role of macrophages and neutrophils. Ageing Res Rev 36:105–116. https://doi.org/10.1016/j.arr.2017.03.008
Tevis KM, Cecchi RJ, Colson YL, Grinstaff MW (2017) Mimicking the tumor microenvironment to regulate macrophage phenotype and assessing chemotherapeutic efficacy in embedded cancer cell/macrophage spheroid models. Acta Biomater 50:271–279. https://doi.org/10.1016/j.actbio.2016.12.037
Zhihua Y, Yulin T, Yibo W, Wei D, Yin C, Jiahao X, Runqiu J, Xuezhong X (2019) Hypoxia decreases macrophage glycolysis and M1 percentage by targeting microRNA-30c and mTOR in human gastric cancer. Cancer Sci 110:2368–2377. https://doi.org/10.1111/cas.14110
Liu F, Bu Z, Zhao F, Xiao D (2018) Increased T-helper 17 cell differentiation mediated by exosome-mediated microRNA-451 redistribution in gastric cancer infiltrated T cells. Cancer Sci 109:65–73. https://doi.org/10.1111/cas.13429
Zhao M, Liu Q, Liu W, Zhou H, Zang X, Lu J (2019) MicroRNA140 suppresses Helicobacter pyloripositive gastric cancer growth by enhancing the antitumor immune response. Mol Med Rep 20:2484–2492. https://doi.org/10.3892/mmr.2019.10475
Zhang Z, Chen S, Fan M, Ruan G, Xi T, Zheng L, Guo L, Ye F, Xing Y (2021) Helicobacter pylori induces gastric cancer via down-regulating miR-375 to inhibit dendritic cell maturation. Helicobacter 26. https://doi.org/10.1111/hel.12813
Ou J, Lei P, Yang Z, Yang M, Luo L, Mo H, Luo G, He J (2021) LINC00152 mediates CD8(+) T-cell infiltration in gastric cancer through binding to EZH2 and regulating the CXCL9, 10/CXCR3 axis. J Mol Histol 52:611–620. https://doi.org/10.1007/s10735-021-09967-z
Xu P, Xu X, Wu X, Zhang L, Meng L, Chen Z, Han W, Yao J, Xu A (2022) CircTMC5 promotes gastric cancer progression and metastasis by targeting miR-361-3p/RABL6. Gastric Cancer 25:64–82. https://doi.org/10.1007/s10120-021-01220-6
Kalluri R (2016) The biology and function of exosomes in cancer. J Clin Investig 126:1208–1215. https://doi.org/10.1172/jci81135
Shao C, Yang F, Miao S, Liu W, Wang C, Shu Y, Shen H (2018) Role of hypoxia-induced exosomes in tumor biology. Mol Cancer 17:120. https://doi.org/10.1186/s12943-018-0869-y
Xu W, Li Z, Zhu X, Xu R, Xu Y (2018) miR-29 Family inhibits resistance to methotrexate and promotes cell apoptosis by targeting COL3A1 and MCL1 in osteosarcoma. Med Sci Monit 24:8812–8821. https://doi.org/10.12659/MSM.911972
Zhao B, Song X, Guan H (2020) CircACAP2 promotes breast cancer proliferation and metastasis by targeting miR-29a/b-3p-COL5A1 axis. Life Sci 244. https://doi.org/10.1016/j.lfs.2019.117179
Liu W, Wei H, Gao Z, Chen G, Liu Y, Gao X, Bai G, He S, Liu T, Xu W et al (2018) COL5A1 may contribute the metastasis of lung adenocarcinoma. Gene 665:57–66. https://doi.org/10.1016/j.gene.2018.04.066
Shi Y, Zheng C, Jin Y, Bao B, Wang D, Hou K, Feng J, Tang S, Qu X, Liu Y et al (2020) Reduced expression of METTL3 promotes metastasis of triple-negative breast cancer by m6A methylation-mediated COL3A1 up-regulation. Front Oncol 10:1126. https://doi.org/10.3389/fonc.2020.01126
Xiao Q, Ge G (2012) Lysyl oxidase, extracellular matrix remodeling and cancer metastasis. Cancer Microenviron 5:261–273. https://doi.org/10.1007/s12307-012-0105-z
Goddard ET, Bozic I, Riddell SR, Ghajar CM (2018) Dormant tumour cells, their niches and the influence of immunity. Nat Cell Biol 20:1240–1249. https://doi.org/10.1038/s41556-018-0214-0
Wang L, Steele I, Kumar JD, Dimaline R, Jithesh PV, Tiszlavicz L, Reisz Z, Dockray GJ, Varro A (2016) Distinct miRNA profiles in normal and gastric cancer myofibroblasts and significance in Wnt signaling. Am J Physiol Gastrointest Liver Physiol 310:G696-704. https://doi.org/10.1152/ajpgi.00443.2015
Tetsuroh Saitoh TM, Katoh Masaru (2002) Frequent up-regulation of WNT5A mRNA in primary gastric cancer. Int J Mol Med 9:515–519
Wang MY, PC, CP Wang, (2019) Targeted regulation of miR-17-5p on TMOD1 promotes the development of cardia cancer. Eur Rev Med Pharmacol Sci 23:6170–6178
Han S, Wang Z, Liu J, Wang HD, Yuan Q (2021) miR-29a-3p-dependent COL3A1 and COL5A1 expression reduction assists sulforaphane to inhibit gastric cancer progression. Biochem Pharmacol 188. https://doi.org/10.1016/j.bcp.2021.114539
Wei Z, Chen L, Meng L, Han W, Huang L, Xu A (2020) LncRNA HOTAIR promotes the growth and metastasis of gastric cancer by sponging miR-1277-5p and upregulating COL5A1. Gastric Cancer 23:1018–1032. https://doi.org/10.1007/s10120-020-01091-3
Aran D, Sirota M, Butte AJ (2015) Systematic pan-cancer analysis of tumour purity. Nat Commun 6:8971. https://doi.org/10.1038/ncomms9971
Mao Y, Feng Q, Zheng P, Yang L, Liu T, Xu Y, Zhu D, Chang W, Ji M, Ren L et al (2018) Low tumor purity is associated with poor prognosis, heavy mutation burden, and intense immune phenotype in colon cancer. Cancer Manag Res 10:3569–3577. https://doi.org/10.2147/CMAR.S171855
Na Ri Shin EHJ, Choi Chang In, Moon Hyun Jung, Kwon Chae Hwa, Chu In Sun, Kim Gwang Ha, Jeon Tae Yong, Kim Dae Hwan, Lee Jae Hyuk, Park Do Youn (2012) Overexpression of Snail is associated with lymph node metastasis and poor prognosis in patients with gastric cancer. BMC Cancer 12:521–536
Poincloux R, Lizarraga F, Chavrier P (2009) Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J Cell Sci 122:3015–3024. https://doi.org/10.1242/jcs.034561
He H, Chen W, Wang X, Wang C, Liu F, Shen Z, Xu J, Gu J, Sun Y (2012) Snail is an independent prognostic predictor for progression and patient survival of gastric cancer. Cancer Sci 103:1296–1303. https://doi.org/10.1111/j.1349-7006.2012.02295.x
de la Pena S, Sampieri CL, Ochoa-Lara M, Leon-Cordoba K, Remes-Troche JM (2014) Expression of the matrix metalloproteases 2, 14, 24, and 25 and tissue inhibitor 3 as potential molecular markers in advanced human gastric cancer. Dis Markers 2014. https://doi.org/10.1155/2014/285906
Wang ZQ, He CY, Hu L, Shi HP, Li JF, Gu QL, Su LP, Liu BY, Li C, Zhu Z (2017) Long noncoding RNA UCA1 promotes tumour metastasis by inducing GRK2 degradation in gastric cancer. Cancer Lett 408:10–21. https://doi.org/10.1016/j.canlet.2017.08.013
Xiaobo Guo ZY, Zhi Qiaoming, Wang Dan, Guo Lei, Li Guimei, Miao Ruizhen, Shi Yulong, Kuang Yuting (2016) Long noncoding RNA OR3A4 promotes metastasis and tumorigenicity in gastric cancer. Oncotarget 7:30276–30294
Tian Y, Xing Y, Zhang Z, Peng R, Zhang L, Sun Y (2020) Bioinformatics analysis of key genes and circRNA-miRNA-mRNA regulatory network in gastric cancer. Biomed Res Int 2020:2862701. https://doi.org/10.1155/2020/2862701
Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP, Tattersall IW et al (2014) Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 25:735–747. https://doi.org/10.1016/j.ccr.2014.04.021
Mizutani Y, Kobayashi H, Iida T, Asai N, Masamune A, Hara A, Esaki N, Ushida K, Mii S, Shiraki Y et al (2019) Meflin-positive cancer-associated fibroblasts inhibit pancreatic carcinogenesis. Cancer Res 79:5367–5381. https://doi.org/10.1158/0008-5472.can-19-0454
Elyada E, Bolisetty M, Laise P, Flynn WF, Courtois ET, Burkhart RA, Teinor JA, Belleau P, Biffi G, Lucito MS et al (2019) Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov 9:1102–1123. https://doi.org/10.1158/2159-8290.CD-19-0094
Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, Baik GH, Shibata W, Diprete B, Betz KS et al (2011) Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell 19:257–272. https://doi.org/10.1016/j.ccr.2011.01.020
Karakasheva TA, Lin EW, Tang Q, Qiao E, Waldron TJ, Soni M, Klein-Szanto AJ, Sahu V, Basu D, Ohashi S et al (2018) IL-6 mediates cross-talk between tumor cells and activated fibroblasts in the tumor microenvironment. Cancer Res 78:4957–4970. https://doi.org/10.1158/0008-5472.CAN-17-2268
Hayakawa Y, Ariyama H, Stancikova J, Sakitani K, Asfaha S, Renz BW, Dubeykovskaya ZA, Shibata W, Wang H, Westphalen CB et al (2015) Mist1 Expressing gastric stem cells maintain the normal and neoplastic gastric epithelium and are supported by a perivascular stem cell niche. Cancer Cell 28:800–814. https://doi.org/10.1016/j.ccell.2015.10.003
Kinoshita H, Hirata Y, Nakagawa H, Sakamoto K, Hayakawa Y, Takahashi R, Nakata W, Sakitani K, Serizawa T, Hikiba Y et al (2013) Interleukin-6 mediates epithelial-stromal interactions and promotes gastric tumorigenesis. PLoS ONE 8. https://doi.org/10.1371/journal.pone.0060914
Wang D, Wang X, Si M, Yang J, Sun S, Wu H, Cui S, Qu X, Yu X (2020) Exosome-encapsulated miRNAs contribute to CXCL12/CXCR4-induced liver metastasis of colorectal cancer by enhancing M2 polarization of macrophages. Cancer Lett 474:36–52. https://doi.org/10.1016/j.canlet.2020.01.005
Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascon S, Hatzios SK, Kagan VE et al (2017) Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171:273–285. https://doi.org/10.1016/j.cell.2017.09.021
Zhang H, Deng T, Liu R, Ning T, Yang H, Liu D, Zhang Q, Lin D, Ge S, Bai M et al (2020) CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol Cancer 19:43. https://doi.org/10.1186/s12943-020-01168-8
Zhou Y, Zhong JH, Gong FS, Xiao J (2019) MiR-141-3p suppresses gastric cancer induced transition of normal fibroblast and BMSC to cancer-associated fibroblasts via targeting STAT4. Exp Mol Pathol 107:85–94. https://doi.org/10.1016/j.yexmp.2018.11.014
Li P, Shan JX, Chen XH, Zhang D, Su LP, Huang XY, Yu BQ, Zhi QM, Li CL, Wang YQ et al (2015) Epigenetic silencing of microRNA-149 in cancer-associated fibroblasts mediates prostaglandin E2/interleukin-6 signaling in the tumor microenvironment. Cell Res 25:588–603. https://doi.org/10.1038/cr.2015.51
Junji Kurashige KM, Sawada Genta, Takahashi Yusuke, Eguchi Hidetoshi, Sugimachi Keishi, Mori Masaki, Yanagihara Kazuyoshi, Yashiro Masakazu, Hirakawa Kosei, Baba Hideo, Mimori Koshi (2015) Epigenetic modulation and repression of miR-200b by cancer-associated fibroblasts contribute to cancer invasion and peritoneal dissemination in gastric cancer. Carcinogenesis 36:133–141. https://doi.org/10.1093/carcin/bgu232
Huang C, Liu J, He L, Wang F, Xiong B, Li Y, Yang X (2021) The long noncoding RNA noncoding RNA activated by DNA damage (NORAD)-microRNA-496-Interleukin-33 axis affects carcinoma-associated fibroblasts-mediated gastric cancer development. Bioengineered 12:11738–11755. https://doi.org/10.1080/21655979.2021.2009412
Liu G, Sun J, Yang ZF, Zhou C, Zhou PY, Guan RY, Sun BY, Wang ZT, Zhou J, Fan J et al (2021) Cancer-associated fibroblast-derived CXCL11 modulates hepatocellular carcinoma cell migration and tumor metastasis through the circUBAP2/miR-4756/IFIT1/3 axis. Cell Death Dis 12:260. https://doi.org/10.1038/s41419-021-03545-7
Xiao K, Yu Z, Li X, Li X, Tang K, Tu C, Qi P, Liao Q, Chen P, Zeng Z et al (2016) Genome-wide analysis of Epstein-Barr virus (EBV) integration and strain in C666–1 and Raji Cells. J Cancer 7:214–224. https://doi.org/10.7150/jca.13150
Fan C, Tang Y, Wang J, Xiong F, Guo C, Wang Y, Xiang B, Zhou M, Li X, Wu X et al (2018) The emerging role of Epstein-Barr virus encoded microRNAs in nasopharyngeal carcinoma. J Cancer 9:2852–2864. https://doi.org/10.7150/jca.25460
Quail DF, Joyce JA (2013) Microenvironmental regulation of tumor progression and metastasis. Nat Med 19:1423–1437. https://doi.org/10.1038/nm.3394
Keir ME, Butte MJ, Freeman GJ, Sharpe AH (2008) PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26:677–704. https://doi.org/10.1146/annurev.immunol.26.021607.090331
Tomohide Yamazaki HA, Iwai Hideyuki, Matsuda Hironori, Aoki Mami, Tanno Yuka, Shin Tahiro, Tsuchiya Haruo, Pardoll Drew M, Okumura Ko, Azuma Miyuki, Yagita Hideo (2002) expression of programmed death 1 ligands by murine T cells and APC. J Immunol 169:5538–5545
Zhong X, Tumang JR, Gao W, Bai C, Rothstein TL (2007) PD-L2 expression extends beyond dendritic cells/macrophages to B1 cells enriched for V(H)11/V(H)12 and phosphatidylcholine binding. Eur J Immunol 37:2405–2410. https://doi.org/10.1002/eji.200737461
Haidong Dong GZ, Tamada Koji, Chen Lieping (1999) B7–H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 5:1365–1369
Lin YM, Sung WW, Hsieh MJ, Tsai SC, Lai HW, Yang SM, Shen KH, Chen MK, Lee H, Yeh KT et al (2015) High PD-L1 expression correlates with metastasis and poor prognosis in oral squamous cell carcinoma. PLoS ONE 10. https://doi.org/10.1371/journal.pone.0142656
Gong Z, Zhang S, Zeng Z, Wu H, Yang Q, Xiong F, Shi L, Yang J, Zhang W, Zhou Y et al (2014) LOC401317, a p53-regulated long non-coding RNA, inhibits cell proliferation and induces apoptosis in the nasopharyngeal carcinoma cell line HNE2. PLoS ONE 9. https://doi.org/10.1371/journal.pone.0110674
Patel JS, Hu M, Sinha G, Walker ND, Sherman LS, Gallagher A, Rameshwar P (2016) Non-coding RNA as mediators in microenvironment-breast cancer cell communication. Cancer Lett 380:289–295. https://doi.org/10.1016/j.canlet.2015.11.016
Miliotis C, Slack FJ (2021) miR-105-5p regulates PD-L1 expression and tumor immunogenicity in gastric cancer. Cancer Lett 518:115–126. https://doi.org/10.1016/j.canlet.2021.05.037
Zheng X, Dong L, Wang K, Zou H, Zhao S, Wang Y, Wang G (2019) MiR-21 Participates in the PD-1/PD-L1 pathway-mediated imbalance of Th17/treg cells in patients after gastric cancer resection. Ann Surg Oncol 26:884–893. https://doi.org/10.1245/s10434-018-07117-6
Chen T, Zhang C, Liu Y, Zhao Y, Lin D, Hu Y, Yu J, Li G (2019) A gastric cancer LncRNAs model for MSI and survival prediction based on support vector machine. BMC Genomics 20:846. https://doi.org/10.1186/s12864-019-6135-x
Wang J, Yu Z, Wang J, Shen Y, Qiu J, Zhuang Z (2020) LncRNA NUTM2A-AS1 positively modulates TET1 and HIF-1A to enhance gastric cancer tumorigenesis and drug resistance by sponging miR-376a. Cancer Med 9:9499–9510. https://doi.org/10.1002/cam4.3544
Spaccarelli N, Rook AH (2015) The use of interferons in the treatment of cutaneous T-cell lymphoma. Dermatol Clin 33:731–745. https://doi.org/10.1016/j.det.2015.05.008
Martinez R, de Villavicencio-Diaz TN, Sanchez A, Ramos Y, Ferro JN, Gonzalez LG, Mendez M, Rodriguez E, Marcos E, Sanchez B et al (2016) Comparative proteomic analysis of growth hormone secretagogue A233 treatment of murine macrophage cells J774A.2 indicates it has a role in antiviral innate response. Biochem Biophys Rep 5:379–387. https://doi.org/10.1016/j.bbrep.2016.01.008
Matalka HAAaKZ, (2011) IFN-g, IL-17 and TGF-b involvement in shaping the tumor microenvironment: the significance of modulating such cytokines in treating malignant solid tumors. Cancer Cell Int 11:33
Mandai M, Hamanishi J, Abiko K, Matsumura N, Baba T, Konishi I (2016) Dual faces of IFNgamma in cancer progression: a role of PD-L1 induction in the determination of pro- and antitumor immunity. Clin Cancer Res 22:2329–2334. https://doi.org/10.1158/1078-0432.CCR-16-0224
Mimura K, Teh JL, Okayama H, Shiraishi K, Kua L-F, Koh V, Smoot DT, Ashktorab H, Oike T, Suzuki Y et al (2018) PD-L1 expression is mainly regulated by interferon gamma associated with JAK-STAT pathway in gastric cancer. Cancer Sci 109:43–53. https://doi.org/10.1111/cas.13424
Moon JW, Kong S-K, Kim BS, Kim HJ, Lim H, Noh K, Kim Y, Choi J-W, Lee J-H, Kim Y-S (2017) IFNγ induces PD-L1 overexpression by JAK2/STAT1/IRF-1 signaling in EBV-positive gastric carcinoma. Sci Rep. https://doi.org/10.1038/s41598-017-18132-0
Gong A-Y, Zhou R, Hu G, Li X, Splinter PL, O’Hara SP, LaRusso NF, Soukup GA, Dong H, Chen X-M (2009) MicroRNA-513 regulates B7–H1 translation and is involved in IFN-γ-induced B7–H1 expression in cholangiocytes. J Immunol 182:1325–1333. https://doi.org/10.4049/jimmunol.182.3.1325
Yuan J, Tan L, Yin Z, Zhu W, Tao K, Wang G, Shi W, Gao J (2019) MIR17HG-miR-18a/19a axis, regulated by interferon regulatory factor-1, promotes gastric cancer metastasis via Wnt/β-catenin signalling. Cell Death Dis. https://doi.org/10.1038/s41419-019-1685-z
Wei MF, Gu ZS, Zheng LL, Zhao MX, Wang XJ (2020) Long non-coding RNA GAS5 promotes natural killer cell cytotoxicity against gastric cancer by regulating miR-18a. Neoplasma 67:1085–1093. https://doi.org/10.4149/neo_2020_191014N1034
Yamaoka Y, Kim HJ, Song DE, Lim SY, Lee S-H, Kang JL, Lee SJ, Benveniste EN, Choi Y-H (2011) Loss of the promyelocytic leukemia protein in gastric cancer: implications for IP-10 expression and tumor-infiltrating lymphocytes. PLoS ONE. https://doi.org/10.1371/journal.pone.0026264
Yee D, Shah KM, Coles MC, Sharp TV, Lagos D (2017) MicroRNA-155 induction via TNF-α and IFN-γ suppresses expression of programmed death ligand-1 (PD-L1) in human primary cells. J Biol Chem 292:20683–20693. https://doi.org/10.1074/jbc.M117.809053
Hartley G, Regan D, Guth A, Dow S (2017) Regulation of PD-L1 expression on murine tumor-associated monocytes and macrophages by locally produced TNF-α. Cancer Immunol Immunother 66:523–535. https://doi.org/10.1007/s00262-017-1955-5
Wang X, Yang L, Huang F, Zhang Q, Liu S, Ma L, You Z (2017) Inflammatory cytokines IL-17 and TNF-α up-regulate PD-L1 expression in human prostate and colon cancer cells. Immunol Lett 184:7–14. https://doi.org/10.1016/j.imlet.2017.02.006
Yan Liu LL, Xiaoxu Wu, Qi Haiyan, Gao Yan, Li Yanqi, Chen Da (2021) MSC-AS1 induced cell growth and inflammatory mediators secretion through sponging miR-142-5p/DDX5 in gastric carcinoma. Aging (Albany NY) 13:10387–10395
Guo T, Zhang Y, Qu X, Che X, Li C, Fan Y, Wan X, Ma R, Hou K, Zhou H et al (2018) miR-200a enhances TRAIL-induced apoptosis in gastric cancer cells by targeting A20. Cell Biol Int 42:506–514. https://doi.org/10.1002/cbin.10924
Zhou C, Li X, Zhang X, Liu X, Tan Z, Yang C, Zhang J (2013) microRNA-372 maintains oncogene characteristics by targeting TNFAIP1 and affects NFκB signaling in human gastric carcinoma cells. Int J Oncol 42:635–642. https://doi.org/10.3892/ijo.2012.1737
Zhong L, Huot J, Simard MJ (2018) p38 activation induces production of miR-146a and miR-31 to repress E-selectin expression and inhibit transendothelial migration of colon cancer cells. Sci Rep 8:2334. https://doi.org/10.1038/s41598-018-20837-9
Potente M, Gerhardt H, Carmeliet P (2011) Basic and therapeutic aspects of angiogenesis. Cell 146:873–887. https://doi.org/10.1016/j.cell.2011.08.039
Ferrara N (2009) Vascular endothelial growth factor. Arterioscler Thromb Vasc Biol 29:789–791. https://doi.org/10.1161/ATVBAHA.108.179663
Kazerounian S, Yee KO, Lawler J (2008) Thrombospondins in cancer. Cell Mol Life Sci 65:700–712. https://doi.org/10.1007/s00018-007-7486-z
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. https://doi.org/10.1016/j.cell.2011.02.013
Wu ZH, Lin C, Liu CC, Jiang WW, Huang MZ, Liu X, Guo WJ (2018) MiR-616-3p promotes angiogenesis and EMT in gastric cancer via the PTEN/AKT/mTOR pathway. Biochem Biophys Res Commun 501:1068–1073. https://doi.org/10.1016/j.bbrc.2018.05.109
Yang H, Zhang H, Ge S, Ning T, Bai M, Li J, Li S, Sun W, Deng T, Zhang L et al (2018) Exosome-derived miR-130a activates angiogenesis in gastric cancer by targeting C-MYB in vascular endothelial cells. Mol Ther 26:2466–2475. https://doi.org/10.1016/j.ymthe.2018.07.023
Deng T, Zhang H, Yang H, Wang H, Bai M, Sun W, Wang X, Si Y, Ning T, Zhang L et al (2020) Exosome miR-155 derived from gastric carcinoma promotes angiogenesis by targeting the c-MYB/VEGF axis of endothelial cells. Mol Ther Nucleic Acids 19:1449–1459. https://doi.org/10.1016/j.omtn.2020.01.024
Zhou Z, Zhang H, Deng T, Ning T, Liu R, Liu D, Bai M, Ying G, Ba Y (2019) Exosomes carrying microRNA-155 target forkhead box O3 of endothelial cells and promote angiogenesis in gastric cancer. Mol Ther Oncolytics 15:223–233. https://doi.org/10.1016/j.omto.2019.10.006
Bai M, Li J, Yang H, Zhang H, Zhou Z, Deng T, Zhu K, Ning T, Fan Q, Ying G et al (2019) miR-135b delivered by gastric tumor exosomes inhibits FOXO1 expression in endothelial cells and promotes angiogenesis. Mol Ther 27:1772–1783. https://doi.org/10.1016/j.ymthe.2019.06.018
Xie M, Yu T, Jing X, Ma L, Fan Y, Yang F, Ma P, Jiang H, Wu X, Shu Y et al (2020) Exosomal circSHKBP1 promotes gastric cancer progression via regulating the miR-582-3p/HUR/VEGF axis and suppressing HSP90 degradation. Mol Cancer 19:112. https://doi.org/10.1186/s12943-020-01208-3
Zhang Z, Harrison PM, Liu Y, Gerstein M (2003) Millions of years of evolution preserved: a comprehensive catalog of the processed pseudogenes in the human genome. Genome Res 13:2541–2558. https://doi.org/10.1101/gr.1429003
Hu X, Yang L, Mo YY (2018) Role of pseudogenes in tumorigenesis. Cancers (Basel). https://doi.org/10.3390/cancers10080256
Wang Y, Han G, Wang K, Liu G, Wang R, Xiao H, Li X, Hou C, Shen B, Guo R et al (2014) Tumor-derived GM-CSF promotes inflammatory colon carcinogenesis via stimulating epithelial release of VEGF. Cancer Res 74:716–726. https://doi.org/10.1158/0008-5472.CAN-13-1459
Huang HW, Chen CY, Huang YH, Yeh CT, Wang CS, Chang CC, Lin KH (2022) CMAHP promotes metastasis by reducing ubiquitination of Snail and inducing angiogenesis via GM-CSF overexpression in gastric cancer. Oncogene 41:159–172. https://doi.org/10.1038/s41388-021-02087-8
Lisa M, Coussens ZW (2002) Inflammation and cancer. Nature 420:860–867
Walczak H (2011) TNF and ubiquitin at the crossroads of gene activation, cell death, inflammation, and cancer. Immunol Rev 244:9–28
Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454:436–444. https://doi.org/10.1038/nature07205
Grivennikov SI, Karin M (2010) Inflammation and oncogenesis: a vicious connection. Curr Opin Genet Dev 20:65–71. https://doi.org/10.1016/j.gde.2009.11.004
Stephens PJ, McBride DJ, Lin ML, Varela I, Pleasance ED, Simpson JT, Stebbings LA, Leroy C, Edkins S, Mudie LJ et al (2009) Complex landscapes of somatic rearrangement in human breast cancer genomes. Nature 462:1005–1010. https://doi.org/10.1038/nature08645
Pleasance ED, Stephens PJ, O’Meara S, McBride DJ, Meynert A, Jones D, Lin ML, Beare D, Lau KW, Greenman C et al (2010) A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature 463:184–190. https://doi.org/10.1038/nature08629
Pleasance ED, Cheetham RK, Stephens PJ, McBride DJ, Humphray SJ, Greenman CD, Varela I, Lin ML, Ordonez GR, Bignell GR et al (2010) A comprehensive catalogue of somatic mutations from a human cancer genome. Nature 463:191–196. https://doi.org/10.1038/nature08658
Ushijima T, Hattori N (2012) Molecular pathways: involvement of Helicobacter pylori-triggered inflammation in the formation of an epigenetic field defect, and its usefulness as cancer risk and exposure markers. Clin Cancer Res 18:923–929. https://doi.org/10.1158/1078-0432.CCR-11-2011
Niwa T, Tsukamoto T, Toyoda T, Mori A, Tanaka H, Maekita T, Ichinose M, Tatematsu M, Ushijima T (2010) Inflammatory processes triggered by Helicobacter pylori infection cause aberrant DNA methylation in gastric epithelial cells. Cancer Res 70:1430–1440. https://doi.org/10.1158/0008-5472.CAN-09-2755
Zhong L, Simard MJ, Huot J (2018) Endothelial microRNAs regulating the NF-κB pathway and cell adhesion molecules during inflammation. FASEB J : Official Pub Federation of Am Societies For Experimental Biol 32:4070–4084. https://doi.org/10.1096/fj.201701536R
Han TS, Voon DC, Oshima H, Nakayama M, Echizen K, Sakai E, Yong ZWE, Murakami K, Yu L, Minamoto T et al (2019) Interleukin 1 up-regulates MicroRNA 135b to promote inflammation-associated gastric carcinogenesis in mice. Gastroenterology 156(1140–1155). https://doi.org/10.1053/j.gastro.2018.11.059
Ting Li HG, Zhao Xiaodi, Jin Jiang, Zhang Lifeng, Li Hong, Yuanyuan Lu, Nie Yongzhan, Kaichun Wu, Shi Yongquan, Fan Daiming (2017) Gastric cancer cell proliferation and survival is enabled by a cyclophilin B/STAT3/miR-520d-5p Signaling Feedback Loop. Cancer Res 77:1227–1240
Shao L, Chen Z, Soutto M, Zhu S, Lu H, Romero-Gallo J, Peek R, Zhang S, El-Rifai W (2019) Helicobacter pylori-induced miR-135b-5p promotes cisplatin resistance in gastric cancer. FASEB J 33:264–274. https://doi.org/10.1096/fj.201701456RR
Kong D, Piao YS, Yamashita S, Oshima H, Oguma K, Fushida S, Fujimura T, Minamoto T, Seno H, Yamada Y et al (2012) Inflammation-induced repression of tumor suppressor miR-7 in gastric tumor cells. Oncogene 31:3949–3960. https://doi.org/10.1038/onc.2011.558
Han T, Jing X, Bao J, Zhao L, Zhang A, Miao R, Guo H, Zhou B, Zhang S, Sun J et al (2020) H. pylori infection alters repair of DNA double-strand breaks via SNHG17. J Clin Invest 130:3901–3918. https://doi.org/10.1172/JCI125581
Jiang T, Xia Y, Lv J, Li B, Li Y, Wang S, Xuan Z, Xie L, Qiu S, He Z et al (2021) A novel protein encoded by circMAPK1 inhibits progression of gastric cancer by suppressing activation of MAPK signaling. Mol Cancer 20:66. https://doi.org/10.1186/s12943-021-01358-y
Jiang XJWJ, Deng XY, Li XL, Li XY, Zeng ZY, Xiong W, Li GY, Xiong F, Guo C (2018) Immunotherapy targeted to immune checkpoint: a revolutionary breakthrough in cancer therapy. Prog Biochem Biophys 11:1178–1186. https://doi.org/10.16476/j.pibb.2018.0264
Jiang X, Shapiro DJ (2014) The immune system and inflammation in breast cancer. Mol Cell Endocrinol 382:673–682. https://doi.org/10.1016/j.mce.2013.06.003
Wang Y-A, Li X-L, Mo Y-Z, Fan C-M, Tang L, Xiong F, Guo C, Xiang B, Zhou M, Ma J et al (2018) Effects of tumor metabolic microenvironment on regulatory T cells. Mol Cancer 17:168. https://doi.org/10.1186/s12943-018-0913-y
Benencia F, Muccioli M, Alnaeeli M (2014) Perspectives on reprograming cancer-associated dendritic cells for anti-tumor therapies. Front Oncol 4:72. https://doi.org/10.3389/fonc.2014.00072
Chanmee T, Ontong P, Konno K, Itano N (2014) Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 6:1670–1690. https://doi.org/10.3390/cancers6031670
Ishida YAY, Shibahara K, Honjo T (1992) Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. Eur Mol Biol Org J 11:3887–3895
Liang Y, Liu Y, Zhang Q, Zhang H, Du J (2021) Tumor-derived extracellular vesicles containing microRNA-1290 promote immune escape of cancer cells through the Grhl2/ZEB1/PD-L1 axis in gastric cancer. Transl Res 231:102–112. https://doi.org/10.1016/j.trsl.2020.12.003
Li H, Zhao C, Zhao H, Liu G, Mao H, Liu Y (2021) Elevated linc00936 or silenced microRNA-425-3p inhibits immune escape of gastric cancer cells via elevation of ZC3H12A. Int Immunopharmacol 95. https://doi.org/10.1016/j.intimp.2021.107559
Sun L, Li J, Yan W, Yao Z, Wang R, Zhou X, Wu H, Zhang G, Shi T, Chen W (2021) H19 promotes aerobic glycolysis, proliferation, and immune escape of gastric cancer cells through the microRNA-519d-3p/lactate dehydrogenase A axis. Cancer Sci 112:2245–2259. https://doi.org/10.1111/cas.14896
Han YJ, Zhang J, Lee JH, Mason JM, Karginova O, Yoshimatsu TF, Hao Q, Hurley I, Brunet LP, Prat A et al (2021) The BRCA1 pseudogene negatively regulates antitumor responses through inhibition of innate immune defense mechanisms. Cancer Res 81:1540–1551. https://doi.org/10.1158/0008-5472.CAN-20-1959
Gao C, Xu YJ, Qi L, Bao YF, Zhang L, Zheng L (2021) CircRNA VIM silence synergizes with sevoflurane to inhibit immune escape and multiple oncogenic activities of esophageal cancer by simultaneously regulating miR-124/PD-L1 axis. Cell Biol Toxicol.https://doi.org/10.1007/s10565-021-09613-0
Shah MY, Ferrajoli A, Sood AK, Lopez-Berestein G, Calin GA (2016) microRNA therapeutics in cancer — an emerging concept. EBioMedicine 12:34–42. https://doi.org/10.1016/j.ebiom.2016.09.017
Donlic A, Hargrove AE (2018) Targeting RNA in mammalian systems with small molecules. WIREs RNA. https://doi.org/10.1002/wrna.1477
Shi LWZ, Geng X, Zhang Y, Xue Z (2020) Exosomal miRNA-34 from cancer-associated fibroblasts inhibits growth and invasion of gastric cancer cells in vitro and in vivo. Aging (Albany NY) 12:8549–8564
Li Z, Suo B, Long G, Gao Y, Song J, Zhang M, Feng B, Shang C, Wang D (2020) Exosomal miRNA-16-5p derived from M1 macrophages enhances T cell-dependent immune response by regulating PD-L1 in gastric cancer. Front Cell and Dev Biol. https://doi.org/10.3389/fcell.2020.572689
Wang X, Zhang H, Bai M, Ning T, Ge S, Deng T, Liu R, Zhang L, Ying G, Ba Y (2018) Exosomes serve as nanoparticles to deliver anti-miR-214 to reverse chemoresistance to cisplatin in gastric cancer. Mol Ther 26:774–783. https://doi.org/10.1016/j.ymthe.2018.01.001
Ji R, Zhang X, Gu H, Ma J, Wen X, Zhou J, Qian H, Xu W, Qian J, Lin J (2019) miR-374a-5p: a new target for diagnosis and drug resistance therapy in gastric cancer. Molecular Therapy - Nucleic Acids 18:320–331. https://doi.org/10.1016/j.omtn.2019.07.025
Kanasty R, Dorkin JR, Vegas A, Anderson D (2013) Delivery materials for siRNA therapeutics. Nat Mater 12:967–977. https://doi.org/10.1038/nmat3765
Ling H (2016) Non-coding RNAs: therapeutic strategies and delivery systems non-coding RNAs in colorectal cancer. 229-237
Van Roosbroeck K, Calin GA (2017) Cancer hallmarks and microRNAs: the therapeutic connection MiRNA and cancer. 119-149
Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, Lee JJ, Kalluri R (2017) Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 546:498–503. https://doi.org/10.1038/nature22341
Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, Lovat F, Fadda P, Mao C, Nuovo GJ et al (2012) MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.1209414109
Matsui M, Corey DR (2016) Non-coding RNAs as drug targets. Nat Rev Drug Discovery 16:167–179. https://doi.org/10.1038/nrd.2016.117
Albanese M, Chen YA, Huls C, Gartner K, Tagawa T, Mejias-Perez E, Keppler OT, Gobel C, Zeidler R, Shein M et al (2021) MicroRNAs are minor constituents of extracellular vesicles that are rarely delivered to target cells. PLoS Genet 17. https://doi.org/10.1371/journal.pgen.1009951
Xu G, Zhang B, Ye J, Cao S, Shi J, Zhao Y, Wang Y, Sang J, Yao Y, Guan W, et al (2019) Exosomal miRNA-139 in cancer-associated fibroblasts inhibits gastric cancer progression by repressing MMP11 expression. Int J Biol Sci 15: 2320–2329. https://doi.org/10.7150/ijbs.33750
Li Q, Li B, Li Q, Wei S, He Z, Huang X, Wang L, Xia Y, Xu Z, Li Z, et al (2018) Exosomal miR-21-5p derived from gastric cancer promotes peritoneal metastasis via mesothelial-to-mesenchymal transition. Cell Death Dis 9: 854. https://doi.org/10.1038/s41419-018-0928-8
Acknowledgements
Thanks for the assistance and support of Y. X. Yang, J. R. Chen, Y. Y. Ding, and S. X. Lin. Thanks for the figure created with BioRender.com. Thanks for the editor and all reviewers for their comments.
Funding
This study was supported in part by Grants from the Programs of National Natural Science Foundation of China (no. 81572372, no. 81974373), National Precision Medicine Research Program (2017YFC0908304), National Key Research and Development Program (MOST-2016YFC1303200), and Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-009A).
Author information
Authors and Affiliations
Contributions
Jingyu Deng provided direction. Liqiao Chen drafted and revised the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval and consent to participate
The protocol of this research and the license for usage of clinical data were given by the Institutional Research Ethics Committee of Tianjin Medical University Cancer Institute and Hospital (Tianjin, China) (No. bc2019087).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, L., Deng, J. Role of non-coding RNA in immune microenvironment and anticancer therapy of gastric cancer. J Mol Med 100, 1703–1719 (2022). https://doi.org/10.1007/s00109-022-02264-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-022-02264-6