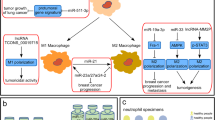
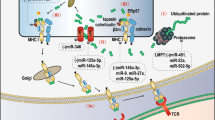
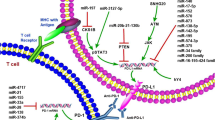
Abstract
Cancer immunology is the study of interaction between cancer cells and immune system by the application of immunology principle and theory. With the recent approval of several new drugs targeting immune checkpoints in cancer, cancer immunology has become a very attractive field of research and is thought to be the new hope to conquer cancer. This chapter introduces the aberrant expression and function of noncoding RNAs, mainly microRNAs and long noncoding RNAs, in tumor-infiltrating immune cells, and their significance in tumor immunity. It also illustrates how noncoding RNAs are shuttled between tumor cells and immune cells in tumor microenvironments via exosomes or other microvesicles to modulate tumor immunity.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Gross L. Intradermal immunization of C3H mice against a sarcoma that originated in an animal of the same line. Cancer Res. 1942;3(5):326–33.
Burnet M. Cancer—a biological approach. I. The processes of control. Br Med J. 1957;1(5022):779–86.
Mayordomo JI, Zorina T, Storkus WJ, et al. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat Med. 1995;1(12):1297–302.
Flamand V, Sornasse T, Thielemans K, et al. Murine dendritic cells pulsed in vitro with tumor antigen induce tumor resistance in vivo. Eur J Immunol. 1994;24(3):605–10. doi:10.1002/eji.1830240317.
Celluzzi CM, Mayordomo JI, Storkus WJ, et al. Peptide-pulsed dendritic cells induce antigen-specific CTL-mediated protective tumor immunity. J Exp Med. 1996;183(1):283–7.
Brossart P, Wirths S, Brugger W, Kanz L. Dendritic cells in cancer vaccines. Exp Hematol. 2001;29(11):1247–55.
Steinman RM, Dhodapkar M. Active immunization against cancer with dendritic cells: the near future. Int J Cancer. 2001;94(4):459–73.
Byrne SN, Halliday GM. Dendritic cells: making progress with tumour regression? Immunol Cell Biol. 2002;80(6):520–30. doi:10.1046/j.1440-1711.2002.01122.x.
Parmiani G, Castelli C, Dalerba P, et al. Cancer immunotherapy with peptide-based vaccines: what have we achieved? where are we going? J Natl Cancer Inst. 2002;94(11):805–18.
Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342(6165):1432–3. doi:10.1126/science.342.6165.1432.
Parish CR. Cancer immunotherapy: the past, the present and the future. Immunol Cell Biol. 2003;81(2):106–13. doi:10.1046/j.0818-9641.2003.01151.x.
Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–60. doi:10.1146/annurev.immunol.22.012703.104803.
Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121(1):1–14. doi:10.1111/j.1365-2567.2007.02587.x.
Su S, Liu Q, Chen J, et al. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell. 2014;25(5):605–20. doi:10.1016/j.ccr.2014.03.021.
Fontana L, Pelosi E, Greco P, et al. MicroRNAs 17-5p–20a–106a control monocytopoiesis through AML1 targeting and M-CSF receptor up-regulation. Nat Cell Biol. 2007;9(7):775–87. doi:10.1038/ncb1613.
Costinean S, Zanesi N, Pekarsky Y, et al. Pre-B cell proliferation and lymphoblastic leukemia/high-grade lymphoma in E(mu)-miR155 transgenic mice. Proc Natl Acad Sci U S A. 2006;103(18):7024–9. doi:10.1073/pnas.0602266103.
Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6(11):857–66. doi:10.1038/nrc1997.
Tili E, Croce CM, Michaille JJ. MiR-155: on the crosstalk between inflammation and cancer. Int Rev Immunol. 2009;28(5):264–84. doi:10.1080/08830180903093796.
O’Connell RM, Chaudhuri AA, Rao DS, Baltimore D. Inositol phosphatase SHIP1 is a primary target of miR-155. Proc Natl Acad Sci U S A. 2009;106(17):7113–18. doi:10.1073/pnas.0902636106.
O’Connell RM, Taganov KD, Boldin MP, et al. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci U S A. 2007;104(5):1604–9. doi:10.1073/pnas.0610731104.
Androulidaki A, Iliopoulos D, Arranz A, et al. The kinase Akt1 controls macrophage response to lipopolysaccharide by regulating microRNAs. Immunity. 2009;31(2):220–31. doi:10.1016/j.immuni.2009.06.024.
Ceppi M, Pereira PM, Dunand-Sauthier I, et al. MicroRNA-155 modulates the interleukin-1 signaling pathway in activated human monocyte-derived dendritic cells. Proc Natl Acad Sci U S A. 2009;106(8):2735–40. doi:10.1073/pnas.0811073106.
Luers AJ, Loudig OD, Berman JW. MicroRNAs are expressed and processed by human primary macrophages. Cell Immunol. 2010;263(1):1–8. doi:10.1016/j.cellimm.2010.03.011.
Liu G, Friggeri A, Yang Y, et al. MiR-147, a microRNA that is induced upon Toll-like receptor stimulation, regulates murine macrophage inflammatory responses. Proc Natl Acad Sci U S A. 2009;106(37):15819–24. doi:10.1073/pnas.0901216106.
Tili E, Michaille JJ, Cimino A, et al. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol. 2007;179(8):5082–9.
Sheedy FJ, Palsson-McDermott E, Hennessy EJ, et al. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol. 2009;11(2):141–7. doi:10.1038/ni.1828.
Zhu D, Pan C, Li L, et al. MicroRNA-17/20a/106a modulate macrophage inflammatory responses through targeting signal-regulatory protein α. J Allergy Clin Immunol. 2013;132(2):426–36. doi:10.1016/j.jaci.2013.02.005.
Bezman NA, Cedars E, Steiner DF, et al. Distinct requirements of MicroRNAs in NK cell activation, survival, and function. J Immunol. 2010;185(7):3835–46. doi:10.4049/jimmunol.1000980.
Yamanaka Y, Tagawa H, Takahashi N, et al. Aberrant overexpression of microRNAs activate AKT signaling via down-regulation of tumor suppressors in natural killer-cell lymphoma/leukemia. Blood. 2009;114(15):3265–75. doi:10.1182/blood-2009-06-222794.
Bezman NA, Chakraborty T, Bender T, Lanier LL. MiR-150 regulates the development of NK and iNKT cells. J Exp Med. 2011;208(13):2717–31. doi:10.1084/jem.20111386.
Heinemann A, Zhao F, Pechlivanis S, et al. Tumor suppressive microRNAs miR-34a/c control cancer cell expression of ULBP2, a stress-induced ligand of the natural killer cell receptor NKG2D. Cancer Res. 2012;72(2):460–71. doi:10.1158/0008-5472.CAN-11-1977.
Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457(7229):557–61. doi:10.1038/nature07665.
Wu H, Neilson JR, Kumar P, et al. miRNA profiling of naive, effector and memory CD8 T cells. Plos One. 2007;2(10):e1020. doi:10.1371/journal.pone.0001020.
Landgraf P, Rusu M, Sheridan R, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129(7):1401–14. doi:10.1016/j.cell.2007.04.040.
Xu H, Cheung IY, Guo HF, Cheung NK. MicroRNA miR-29 modulates expression of immunoinhibitory molecule B7-H3: Potential implications for immune based therapy of human solid tumors. Cancer Res. 2009;69(15):6275–81. doi:10.1158/0008-5472.CAN-08-4517.
Sheppard HM, Verdon D, Brooks AE, et al. MicroRNA regulation in human CD8+ T cell subsets--cytokine exposure alone drives miR-146a expression. J Transl Med. 2014;12:292. doi:10.1186/s12967-014-0292-0.
Saki N, Abroun S, Soleimani M, et al. The roles of miR-146a in the differentiation of Jurkat T-lymphoblasts. Hematology. 2014;19(3):141–7. doi:10.1179/1607845413Y.0000000105.
Yang L, Boldin MP, Yu Y, et al. MiR-146a controls the resolution of T cell responses in mice. J Exp Med. 2012;209(9):1655–70. doi:10.1084/jem.20112218.
Lu LF, Boldin MP, Chaudhry A, et al. Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell. 2010;142(6):914–29. doi:10.1016/j.cell.2010.08.012.
Burger ML, Xue L, Sun Y, et al. Premalignant PTEN-deficient thymocytes activate microRNAs miR-146a and miR-146b as a cellular defense against malignant transformation. Blood. 2014;123(26):4089–100. doi:10.1182/blood-2013-11-539411.
Rodriguez A, Vigorito E, Clare S, et al. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316(5824):608–11. doi:10.1126/science.1139253.
Banerjee A, Schambach F, DeJong CS, et al. Micro-RNA-155 inhibits IFN-gamma signaling in CD4+ T cells. Eur J Immunol. 2010;40(1):225–31. doi:10.1002/eji.200939381.
Lu LF, Thai TH, Calado DP, et al. Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity. 2008;30(1):80–91. doi:10.1016/j.immuni.2008.11.010.
Zhao XD, Zhang W, Liang HJ, Ji WY. Overexpression of miR -155 promotes proliferation and invasion of human laryngeal squamous cell carcinoma via targeting SOCS1 and STAT3. Plos One. 2013;8(2), e56395. doi:10.1371/journal.pone.0056395.
Li QJ, Chau J, Ebert PJ, et al. MiR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129(1):147–61. doi:10.1016/j.cell.2007.03.008.
Li G, Yu M, Lee WW, et al. Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat Med. 2012;18(10):1518–24. doi:10.1038/nm.2963.
Nishimura J, Handa R, Yamamoto H, et al. MicroRNA-181a is associated with poor prognosis of colorectal cancer. Oncol Rep. 2012;28(6):2221–6. doi:10.3892/or.2012.2059.
Xiao C, Srinivasan L, Calado DP, et al. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol. 2008;9(4):405–14. doi:10.1038/ni1575.
Sasaki K, Kohanbash G, Hoji A, et al. MiR-17-92 expression in differentiated T cells – implications for cancer immunotherapy. J Transl Med. 2010;8:17. doi:10.1186/1479-5876-8-17.
Taguchi A, Yanagisawa K, Tanaka M, et al. Identification of hypoxia-inducible factor-1 alpha as a novel target for miR-17-92 microRNA cluster. Cancer Res. 2008;68(14):5540–5. doi:10.1158/0008-5472.CAN-07-6460.
Du C, Liu C, Kang J, et al. MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat Immunol. 2009;10(12):1252–9. doi:10.1038/ni.1798.
Hoareau-Aveilla C, Valentin T, Daugrois C, et al. Reversal of microRNA-150 silencing disadvantages crizotinib-resistant NPM-ALK(+) cell growth. J Clin Invest. 2015;125(9):3505–18. doi:10.1172/JCI78488.
de Yebenes VG, Belver L, Pisano DG, et al. MiR-181b negatively regulates activation-induced cytidine deaminase in B cells. J Exp Med. 2008;205(10):2199–206. doi:10.1084/jem.20080579.
Xu X, Ge S, Jia R, et al. Hypoxia-induced miR-181b enhances angiogenesis of retinoblastoma cells by targeting PDCD10 and GATA6. Oncol Rep. 2015;33(6):2789–96. doi:10.3892/or.2015.3900.
Mraz M, Chen L, Rassenti LZ, et al. MiR-150 influences B-cell receptor signaling in chronic lymphocytic leukemia by regulating expression of GAB1 and FOXP1. Blood. 2014;124(1):84–95. doi:10.1182/blood-2013-09-527234.
Manoharan P, Basford JE, Pilcher-Roberts R, et al. Reduced levels of microRNAs miR-124a and miR-150 are associated with increased proinflammatory mediator expression in Kruppel-like factor 2 (KLF2)-deficient macrophages. J Biol Chem. 2014;289(45):31638–46. doi:10.1074/jbc.M114.579763.
Zhou B, Wang S, Mayr C, et al. MiR-150, a microRNA expressed in mature B and T cells, blocks early B cell development when expressed prematurely. Proc Natl Acad Sci U S A. 2007;104(17):7080–5. doi:10.1073/pnas.0702409104.
Dorsett Y, McBride KM, Jankovic M, et al. MicroRNA-155 suppresses activation-induced cytidine deaminase-mediated Myc-Igh translocation. Immunity. 2008;28(5):630–8. doi:10.1016/j.immuni.2008.04.002.
Wang YZ, Tian FF, Yan M, et al. Delivery of an miR155 inhibitor by anti-CD20 single-chain antibody into B cells reduces the acetylcholine receptor-specific autoantibodies and ameliorates experimental autoimmune myasthenia gravis. Clin Exp Immunol. 2014;176(2):207–21. doi:10.1111/cei.12265.
Neilsen PM, Noll JE, Mattiske S, et al. Mutant p53 drives invasion in breast tumors through up-regulation of miR-155. Oncogene. 2013;32(24):2992–3000. doi:10.1038/onc.2012.305.
Yin Q, Wang X, McBride J, et al. B-cell receptor activation induces BIC/miR-155 expression through a conserved AP-1 element. J Biol Chem. 2008;283(5):2654–62. doi:10.1074/jbc.M708218200.
Carpenter S, Aiello D, Atianand MK, et al. A long non-coding RNA mediates both activation and repression of immune response genes. Science. 2013;341(6147):789–92. doi:10.1126/science.1240925.
Krawczyk M, Emerson BM. P50-associated COX-2 extragenic RNA (PACER) activates COX-2 gene expression by occluding repressive NF-kappaB complexes. Elife. 2014;3:e1776.
Liu B, Sun L, Liu Q, et al. A cytoplasmic NF-kappaB interacting long non-coding RNA blocks IkappaB phosphorylation and suppresses breast cancer metastasis. Cancer Cell. 2015;27(3):370–81. doi:10.1016/j.ccell.2015.02.004.
Wang P, Xue Y, Han Y, et al. The STAT3-binding long non-coding RNA lnc-DC controls human dendritic cell differentiation. Science. 2014;344(6181):310–13. doi:10.1126/science.1251456.
Cui H, Xie N, Tan Z, et al. The human long non-coding RNA lnc-IL7R regulates the inflammatory response. Eur J Immunol. 2014;44(7):2085–95. doi:10.1002/eji.201344126.
Rapicavoli NA, Qu K, Zhang J, et al. A mammalian pseudogene lncRNA at the interface of inflammation and anti-inflammatory therapeutics. Elife. 2013;2:e762. doi:10.7554/eLife.00762.
Collier SP, Henderson MA, Tossberg JT, Aune TM. Regulation of the th1 genomic locus from ifng through tmevpg1 by t-bet. J Immunol. 2014;193(8):3959–65. doi:10.4049/jimmunol.1401099.
Gomez JA, Wapinski OL, Yang YW, et al. The NeST long ncRNA controls microbial susceptibility and epigenetic activation of the interferon-γ locus. Cell. 2013;152(4):743–54. doi:10.1016/j.cell.2013.01.015.
Hu G, Tang Q, Sharma S, et al. Expression and regulation of intergenic long non-coding RNAs during T cell development and differentiation. Nat Immunol. 2013;14(11):1190–8. doi:10.1038/ni.2712.
Zhang H, Nestor CE, Zhao S, et al. Profiling of human CD4+ T-cell subsets identifies the TH2-specific non-coding RNA GATA3-AS1. J Allergy Clin Immunol. 2013;132(4):1005–8. doi:10.1016/j.jaci.2013.05.033.
Hwang SS, Kim K, Lee W, Lee GR. Aberrant expression of IFN-gamma in Th2 cells from Th2 LCR-deficient mice. Biochem Biophys Res Commun. 2012;424(3):512–18. doi:10.1016/j.bbrc.2012.06.146.
Spurlock CF, Tossberg JT, Guo Y, et al. Expression and functions of long non-coding RNAs during human T helper cell differentiation. Nat Commun. 2015;6:6932. doi:10.1038/ncomms7932.
Ranzani V, Rossetti G, Panzeri I, et al. The long intergenic non-coding RNA landscape of human lymphocytes highlights the regulation of T cell differentiation by linc-MAF-4. Nat Immunol. 2015;16(3):318–25. doi:10.1038/ni.3093.
Sharma S, Findlay GM, Bandukwala HS, et al. Dephosphorylation of the nuclear factor of activated T cells (NFAT) transcription factor is regulated by an RNA-protein scaffold complex. Proc Natl Acad Sci U S A. 2011;108(28):11381–6. doi:10.1073/pnas.1019711108.
Sehgal L, Mathur R, Braun FK, et al. FAS-antisense 1 lncRNA and production of soluble versus membrane Fas in B-cell lymphoma. Leukemia. 2014;28(12):2376–87. doi:10.1038/leu.2014.126.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Singapore
About this chapter
Cite this chapter
Li, Q., Liu, Q. (2016). Noncoding RNAs in Cancer Immunology. In: Song, E. (eds) The Long and Short Non-coding RNAs in Cancer Biology. Advances in Experimental Medicine and Biology, vol 927. Springer, Singapore. https://doi.org/10.1007/978-981-10-1498-7_9
Download citation
DOI: https://doi.org/10.1007/978-981-10-1498-7_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-1496-3
Online ISBN: 978-981-10-1498-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)