Abstract
Purpose
Stereotactic body radiotherapy (SBRT) is a key treatment modality for lung cancer patients. This study aims to develop a machine learning-based prediction model of complete response for lung oligometastatic cancer patients undergoing SBRT.
Materials and methods
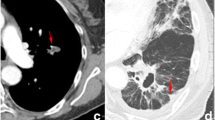
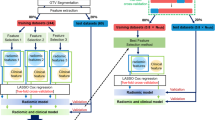
CT images of 80 pulmonary oligometastases from 56 patients treated with SBRT were analyzed. The gross tumor volumes (GTV) were contoured on CT images. Patients that achieved complete response (CR) at 4 months were defined as responders. For each GTV, 107 radiomic features were extracted using the Pyradiomics software. The concordance correlation coefficients (CCC) between the region of interest (ROI)-based radiomics features obtained by the two segmentations were calculated. Pairwise feature interdependencies were evaluated using the Spearman rank correlation coefficient. The association of clinical variables and radiomics features with CR was evaluated with univariate logistic regression. Two supervised machine learning models, the logistic regression (LR) and the classification and regression tree analysis (CART), were trained to predict CR. The models were cross-validated using a five-fold cross-validation. The performance of models was assessed by receiver operating characteristic curve (ROC) and class-specific accuracy, precision, recall, and F1-measure evaluation metrics.
Results
Complete response was associated with four radiomics features, namely the surface to volume ratio (SVR; p = 0.003), the skewness (Skew; p = 0.027), the correlation (Corr; p = 0.024), and the grey normalized level uniformity (GNLU; p = 0.015). No significant relationship between clinical parameters and CR was found. In the validation set, the developed LR and CART machine learning models had an accuracy, precision, and recall of 0.644 and 0.750, 0.644 and 0.651, and 0.635 and 0.754, respectively. The area under the curve for CR prediction was 0.707 and 0.753 for the LR and CART models, respectively.
Conclusion
This analysis demonstrates that radiomics features obtained from pretreatment CT could predict complete response of lung oligometastases following SBRT.




Similar content being viewed by others
References
Timmerman R, Paulus R, Galvin J et al (2010) Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 303(11):1070–1076. https://doi.org/10.1001/jama.2010.261
Virbel G, Le Fèvre C, Noël G, Antoni D (2021) Stereotactic body radiotherapy for patients with lung oligometastatic disease: a five-year systematic review. Cancers 13(14):3623. https://doi.org/10.3390/cancers13143623
Ricardi U, Filippi AR, Guarneri A, Ragona R, Mantovani C, Giglioli F et al (2012) Stereotactic body radiation therapy for lung metastases. Lung Cancer 75:77–81. https://doi.org/10.1016/j.lungcan.2011.04.021
Okunieff P, Petersen AL, Philip A, Milano MT, Katz AW, Boros L et al (2006) Stereotactic Body Radiation Therapy (SBRT) for lung metastases. Acta Oncol 45:808–817. https://doi.org/10.1080/02841860600908954
Nuyttens JJ, van der Voort van Zyp NCMG, Verhoef C, Maat A, van Klaveren RJ, van der Holt B et al (2015) Stereotactic body radiation therapy for oligometastases to the lung: a phase 2 study. Int J Radiat Oncol Biol Phys 91:337–343. https://doi.org/10.1016/j.ijrobp.2014.10.021
Palma DA, Nguyen TK, Louie AV et al (2019) Measuring the integration of stereotactic ablative radiotherapy plus surgery for early-stage non-small cell lung cancer: a phase 2 clinical trial. JAMA Oncol 5:681–688. https://doi.org/10.1001/jamaoncol.2018.6993
Frakulli R, Salvi F, Balestrini D et al (2015) Stereotactic radiotherapy in the treatment of lung metastases from bone and soft-tissue sarcomas. Anticancer Res 35(10):5581–5586
Lin Q, Zhou N, Zhu X et al (2022) Outcomes of SBRT for lung oligo-recurrence of non-small cell lung cancer: a retrospective analysis. J Radiat Res 63(2):272–280. https://doi.org/10.1093/jrr/rrab118
Salama JK, Hasselle MD, Chmura SJ et al (2012) Stereotactic body radiotherapy for multisite extracranial oligometastases: final report of a dose escalation trial in patients with 1 to 5 sites of metastatic disease. Cancer 118(11):2962–2970. https://doi.org/10.1002/cncr.26611
Tanadini-Lang S, Rieber J, Filippi AR et al (2017) Nomogram based overall survival prediction in stereotactic body radiotherapy for oligo-metastatic lung disease. Radiother Oncol 123:182–188. https://doi.org/10.1016/j.radonc.2017.01.003
Baker S, Bakunina K, Duijm M et al (2020) Development and external validation of a nomogram to predict overall survival following stereotactic body radiotherapy for early-stage lung cancer. Radiat Oncol 15(1):89. https://doi.org/10.1186/s13014-020-01537-z
Ye L, Shi S, Zeng Z et al (2018) Nomograms for predicting disease progression in patients of Stage I non-small cell lung cancer treated with stereotactic body radiotherapy. Jpn J Clin Oncol 48(2):160–166. https://doi.org/10.1093/jjco/hyx179
Lambin P, Rios-Velazquez E, Leijenaar R et al (2012) Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 48:441–446. https://doi.org/10.1016/j.ejca.2011.11.036
Gabelloni M, Faggioni L, Fusco R et al (2023) Radiomics in lung metastases: a systematic review. J Pers 13:225. https://doi.org/10.3390/jpm13020225
Franceschini D, Cozzi L, De Rose F et al (2020) A radiomic approach to predicting nodal relapse and disease-specific survival in patients treated with stereotactic body radiation therapy for early-stage non-small cell lung cancer. Strahlenther Onkol 196(10):922–931. https://doi.org/10.1007/s00066-019-01542-6
Lafata KJ, Hong JC, Geng R et al (2019) Association of pre-treatment radiomic features with lung cancer recurrence following stereotactic body radiation therapy. Phys Med Biol 64(2):25007. https://doi.org/10.1088/1361-6560/aaf5a5
Li Q, Kim J, Balagurunathan Y et al (2017) CT imaging features associated with recurrence in non-small cell lung cancer patients after stereotactic body radiotherapy. Radiat Oncol 12(1):158. https://doi.org/10.1186/s13014-017-0892-y
Oikonomou A, Khalvati F, Tyrrell PN et al (2018) Radiomics analysis at PET/CT contributes to prognosis of recurrence and survival in lung cancer treated with stereotactic body radiotherapy. Sci Rep 8(1):4003. https://doi.org/10.1038/s41598-018-22357-y
Huynh E, Coroller TP, Narayan V et al (2016) CT-based radiomic analysis of stereotactic body radiation therapy patients with lung cancer. Radiother Oncol 120(2):258–266. https://doi.org/10.1016/j.radonc.2016.05.024
Li H, Galperin-Aizenberg M, Pryma D et al (2018) Unsupervised machine learning of radiomic features for predicting treatment response and overall survival of early stage non-small cell lung cancer patients treated with stereotactic body radiation therapy. Radiother Oncol 129(2):218–226. https://doi.org/10.1016/j.radonc.2018.06.025
Li Q, Kim J, Balagurunathan Y et al (2017) Imaging features from pretreatment CT scans are associated with clinical outcomes in non-small cell lung cancer patients treated with stereotactic body radiotherapy. Med Phys 44(8):4341–4349. https://doi.org/10.1002/mp.12309
Bousabarah K, Temming S, Hoevels M et al (2019) Radiomic analysis of planning computed tomograms for predicting radiation-induced lung injury and outcome in lung cancer patients treated with robotic stereotactic body radiation therapy. Strahlenther Onkol 195:830–842. https://doi.org/10.1007/s00066-019-01452-7
van Timmeren JE, Carvalho S, Leijenaar RTH et al (2019) Challenges and caveats of a multi-center retrospective radiomics study: an example of early treatment response assessment for NSCLC patients using FDG-PET/CT radiomics. PLoS ONE 14(6):e217536. https://doi.org/10.1371/journal.pone.0217536
Cheung BMF, Lau KS, Lee VHF et al (2021) Computed tomography-based radiomic model predicts radiological response following stereotactic body radiation therapy in early-stage non-small-cell lung cancer and pulmonary oligo-metastases. Radiat Oncol J 39(4):254–264. https://doi.org/10.3857/roj.2021.00311
Deodato F, Macchia G, Cilla S et al (2019) Dose escalation in extracranial stereotactic ablative radiotherapy (DESTROY-1): A multiarm Phase I trial. Br J Radiol 91:20180422. https://doi.org/10.1259/bjr.20180422
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216. https://doi.org/10.1093/jnci/92.3.205
Van Griethuysen JJ, Fedorov A, Parmar C et al (2017) Computational radiomics system to decode the radiographic phenotype. Can Res 77(21):e104–e107. https://doi.org/10.1158/0008-5472.CAN-17-0339
Zwanenburg A, Vallieres M, Abdalah MA et al (2020) The image biomarker standardization initiative: standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology 295(2):328–338. https://doi.org/10.1148/radiol.2020191145
de Sousa VML, Carvalho L (2018) Heterogeneity in lung cancer. Pathobiology 85(1–2):96–107. https://doi.org/10.1159/000487440
Ferreira Junior JR, Koenigkam-Santos M, de Vita Graves C et al (2021) Quantifying intratumor heterogeneity of lung neoplasms with radiomics. Clin Imaging 74:27–30. https://doi.org/10.1016/j.clinimag.2020.12.017
Dou TH, Coroller TP, van Griethuysen JJM et al (2018) Peritumoral radiomics features predict distant metastasis in locally advanced NSCLC. PLoS ONE 13(11):e206108. https://doi.org/10.1371/journal.pone.0206108
Voulaz E, Novellis P, Rossetti F et al (2020) Distinguishing multiple lung primaries from intra-pulmonary metastases and treatment implications. Expert Rev Anticancer Ther 20:985–995. https://doi.org/10.1080/14737140.2020.1823223
Avanzo M, Stancanello J, Pirrone G et al (2020) Radiomics and deep learning in lung cancer. Strahlenther Onkol 196:879–887. https://doi.org/10.1007/s00066-020-01625-9
Davey A, van Herk M, Faivre-Finn C et al (2019) Is tumour sphericity an important prognostic factor in patients with lung cancer? Radiother Oncol 143:73–80. https://doi.org/10.1016/j.radonc.2019.08.003
Salguero FJ, Belderbos JSA, Rossi MMG et al (2013) Microscopic disease extensions as a risk factor for loco-regional recurrence of NSCLC after SBRT. Radiother Oncol 109:26–31. https://doi.org/10.1016/j.radonc.2013.08.028
Coroller TP, Agrawal V, Narayan V et al (2016) Radiomic phenotype features predict pathological response in non-small cell lung cancer. Radiother Oncol 119:480–486. https://doi.org/10.1016/j.radonc.2016.04.004
Chong Y, Kim JH, Lee HY et al (2014) Quantitative CT variables enabling response prediction in neoadjuvant therapy with EGFR-TKIs: are they different from those in neoadjuvant concurrent chemoradiotherapy? Plos One 9:e88598. https://doi.org/10.1371/journal.pone.0088598
Caruso D, Zerunian M, Daffina J et al (2021) Radiomics and functional imaging in lung cancer: the importance of radiological heterogeneity beyond FDG PET/CT and lung biopsy. Eur J Radiol 142:109874. https://doi.org/10.1016/j.ejrad.2021.109874
Weiss GJ, Ganeshan B, Miles KA et al (2014) Noninasive image texture analysis differentiates Kras mutation from pan-wildtype NSCLC and is prognostic. Plos One 9:e100244. https://doi.org/10.1371/journal.pone.0100244
Guan JL, Zhong WZ, An SJ et al (2013) KRAS mutation in patients with lung cancer: a predictor for poor prognosis but not for EGFR-KIs or chemotherapy. Ann Surg Oncol 20:1381–1388. https://doi.org/10.1245/s10434-012-2754-z
Wang M, Han J, Marcar L et al (2017) Radiation resistance in KRAS mutated lung cancer is enabled by stem-like properties mediated by an osteopontin-EGFR pathway. Cancer Res 77:2018–2028. https://doi.org/10.1158/0008-5472.CAN-16-0808
Muenzel D, Engels HP, Bruegel M et al (2012) Intra- and inter-observer variability in measurement of target lesions: implication on response evaluation according to RECIST 1.1. Radiol Oncol 46(1):8–18. https://doi.org/10.2478/v10019-012-0009-z
McErlean A, Panicek DM, Zabor EC et al (2013) Intra- and interobserver variability in CT measurements in oncology. Radiology 269(2):451–459. https://doi.org/10.1148/radiology.13122665
Garau N, Paganelli C, Summers P et al (2020) External validation of radiomics-based predictive models in low-dose CT screening for early lung cancer diagnosis. Med Phys 47(9):4125–4136. https://doi.org/10.1002/mp.14308
Funding
The authors received no specific financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
Concept and design: SC. Conduct: SC, DP, FD, CR, GM. Supervision: AGM. Data acquisition: DP, CR, AP, AA, MB. Statistical analysis: SC. Critical review: SC, FD, GM, AGM. Manuscript drafting, editing: all authors. Revision and final approval: all authors.
Corresponding author
Ethics declarations
Conflict of interest
S. Cilla, D. Pistilli, C. Romano, G. Macchia, A. Pierro, A. Arcelli, M. Buwenge, A.G. Morganti, and F. Deodato declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants or on human tissue were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. The study received approval at the Gemelli Molise Hospital Institutional Review Board. Informed consent: not applicable; patients signed informed consent to the treatment procedure, but the study was retrospective.
Additional information
Two authors share the seniorship: Alessio Giuseppe Morganti and Francesco Deodato.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cilla, S., Pistilli, D., Romano, C. et al. CT-based radiomics prediction of complete response after stereotactic body radiation therapy for patients with lung metastases. Strahlenther Onkol 199, 676–685 (2023). https://doi.org/10.1007/s00066-023-02086-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-023-02086-6