Abstract
Introduction
Data regarding the possible role of heparanase (HPA) in the occurrence of left atrial/left atrial appendage (LA/LAA) thrombus in patients with atrial fibrillation (AF) is lacking. The goal of the present study was to assess the association between plasma levels of HPA and LA/LAA thrombus in AF.
Methods
A total of 687 patients with nonvalvular AF (NVAF) without anticoagulation therapy were included from January 2016 to June 2019. Serum HPA analysis was performed with a commercially available human ELISA kit. Logistic regression models were used to test for association.
Results
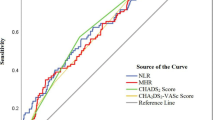
Serum HPA levels were significantly higher in patients with LA/LAA thrombus than in those without LA/LAA thrombus (270.8 [193.4 ± 353.2] pg/mL vs 150.3 [125.2 ± 208.4] pg/mL; P < 0.001). In multivariate analysis, serum HPA remained a significantly independent predictor of LA/LAA thrombus (odds ratio 1.674, 95% confidence interval [CI] 1.339–2.289, P < 0.001). In the receiver operating characteristic (ROC) curve analysis, HPA showed a predictive value with an area under the curve (AUC) of 0.757 (95% CI 0.652–0.810, P < 0.001). The optimal cutoff level for HPA predicting LA/LAA thrombus was 210.7 pg/mL, with a sensitivity of 74.3% and a specificity of 64.8%.
Conclusion
An elevated HPA level was associated with the presence of LA/LAA thrombus in patients with AF. HPA might portend the risk for the prothrombotic state in AF patients.
Zusammenfassung
Hintergrund
Es fehlen Daten zur möglichen Rolle von Heparanase (HPA) beim Auftreten eines Thrombus linksatrial oder im linken Herzohr (LA/LAA) bei Patienten mit Vorhofflimmern (VF). Ziel der vorliegenden Studie war es, den Zusammenhang zwischen den Plasmaspiegel von HPA und einem LA/LAA-Thrombus bei VF zu untersuchen.
Methoden
Von Januar 2016 bis Juni 2019 wurden in die Studie 687 Patienten mit nichtvalvulärem VF (NVVF) ohne Antikoagulationstherapie einbezogen. Eine Serum-HPA-Analyse erfolgte mit einem kommerziell erhältlichen humanen ELISA-Kit. Logistische Regressionsmodelle wurden zur Prüfung des Zusammenhangs eingesetzt.
Ergebnisse
Die Serum-HPA-Spiegel waren bei Patienten mit LA/LAA-Thrombus signifikant höher als bei Patienten ohne LA/LAA-Thrombus (270,8 [193,4 ± 353,2] pg/ml vs. 150,3 [125,2 ± 208,4] pg/ml; p < 0,001). In der multivariaten Analyse blieb die Serum-HPA ein signifikant unabhängiger Prädiktor eines LA/LAA-Thrombus (Odds Ratio 1,674; 95 %-Konfidenzintervall, 95 %-KI: 1,339–2,289; p < 0,001). In der Receiver-Operating-Characteristic(ROC)-Kurvenanalyse zeigte sich für HPA ein prädiktiver Wert mit einem Areal unter der Kurve (AUC) von 0,757 (95 %-KI: 0,652–0,810; p < 0,001). Der optimale Grenzwert für HPA zur Vorhersage eines LA/LAA-Thrombus betrug 210,7 pg/ml, mit einer Sensitivität von 74,3 % und einer Spezifität von 64,8 %.
Schlussfolgerung
Ein erhöhter HPA-Spiegel ging mit dem Vorliegen eines LA/LAA-Thrombus bei Patienten mit VF einher. Der HPA-Wert könnte ein Hinweis auf das Risiko eines prothrombotischen Zustands bei VF-Patienten sein.


Similar content being viewed by others
References
Wolf PA et al (1991) Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 22:983–988
Yarmohammadi H et al (2013) Efficacy of the CHADS 2 scoring system to assess left atrial thrombogenic milieu risk before cardioversion of non-valvular atrial fibrillation. Am J Cardiol 112:678–683
Wasmer K et al (2013) CHADS 2 and CHA 2DS2- VAS c score of patients with atrial fibrillation or flutter and newly detected left atrial thrombus. Clin Res Cardiol 102:139–144
Freeman C, Parish CR (1998) Human platelet heparanase: purification, characterization and catalytic activity. Biochem J 330:1341–1350
Pikas DS et al (1998) Substrate specificity of heparanases from human hepatoma and platelets. J Biol Chem 273:18770–18777
Nadir Y, Brenner B (2014) Heparanase multiple effects in cancer. Thromb Res 133:904
Crispel Y et al (2017) Involvement of the heparanese procoagulant domain in bleeding and wound healing. J Thromb Haemost 15:1463–1472
Nadir Y, Brenner B (2012) Heparanase‑A link between coagulation, angiogenesis and cancer. Rambam Maimonides Med J 31:e2
Parish CR et al (2011) Heparanase:a key enzyme involved in cell invazion. Biochim Biophys Acta 1471:99–108
Nadir Y, Brenner B (2012) Heparanase procoagulant activity. Thromb Res 129:76–79
Nadir Y et al (2010) Heparanase enhances the generation of activated factor X in the presence of tissue factor and activated factor VII. Haematologica 95:1927–1934
Peled E et al (2016) Heparanase procoagulant activity as a predictor of wound necrosis following diabetic foot amputation. Thromb Res 139:148–153
Hu Y et al (2017) Serum heparanase concentration and heperanse activity in patients with retinal vein occlusion. Acta Ophthalmol 95:e62–e66
Pisters R et al (2012) Stroke and thromboembolism in atrial fibrillation. Circ J 76:2289–2304
Nadir Y et al (2014) Heparanase procoagulant activity is elevated and predicts survival in non-small cell lung cancer patients. Thromb Res 134:639–642
Watson T et al (2009) Mechanisms of thrombogenesis in atrial fibrillation: Virchow’s triad revisited. Lancet 373:155–166
Khan AA, Lip GYH (2019) The prothrombotic state in atrial fibrillation:pathophysiological and management implications. Cardiovasc Res 115(1):31–45. https://doi.org/10.1093/cvr/cvy272
Wu N et al (2015) Association of hemostatic markers with atrial fibrillation: a meta-analysis and meta-regression. PLoS ONE 10(4):e124716. https://doi.org/10.1371/journal.pone.0124716
Wysokinski WE et al (2018) Association between P‑selectin levels and left atrial blood stasis in patients with nonvalvular atrial fibrillation. Thromb Res 172:4–8. https://doi.org/10.1016/j.thromres.2018.10.009
Cohoon KP et al (2016) Association of soluble CD40 Ligand with duration of atrial fibrillation and with intensity of spontaneous echocardiographic contrast. JACC Clin Electrophysiol 2(5):623–632. https://doi.org/10.1016/j.jacep.2016.03.006
Wysokinski WE et al (2010) Predicting left atrial thrombi in atrial fibrillation. Am Heart J 159(4):665–671. https://doi.org/10.1016/j.ahj.2009.12.043
Fu R et al (2011) A study of blood soluble P‑selectin, fibrinogen, and von Willebrand factor levels in idiopathic and lone atrial fibrillation. Europace 13(1):31–36. https://doi.org/10.1093/europace/euq346
Yao Y et al (2018) Elevated homocysteine increases the risk of left atrial/left atrial appendage thrombus in non-valvular atrial fibrillation with low CHA2DS2-VASc score. Europace 20(7):1093–1098. https://doi.org/10.1093/europace/eux189
Cui H et al (2016) Heparanase expression upregulates platelet adhesion activity and thrombogenicity. Oncotarget 7(26):39486–39496. https://doi.org/10.18632/oncotarget.8960
Nadir Y et al (2011) An assay to evaluate heparanase procoagulant activity. Thromb Res 128(4):e3–e8. https://doi.org/10.1016/j.thromres.2011.03.008
Matan M et al (2013) Heparanase procoagulant activity is elevated in women using oral contraceptives. Hum Reprod 28(9):2372–2380. https://doi.org/10.1093/humrep/det257
Peled E et al (2012) Increased heparanase level and procoagulant activity in orthopedic surgery patients receiving prophylactic dose of enoxaparin. Thromb Res 130(1):129–134. https://doi.org/10.1016/j.thromres.2011.09.021
Bayam E et al (2018) The relationship between heparanase levels, thrombus burden and thromboembolism in patients receiving unfractionated heparin treatment for prosthetic valve thrombosis. Thromb Res 171:103–110. https://doi.org/10.1016/j.thromres.2018.09.061
Gurbuz AS et al (2019) Heparanase is a predictive marker for high thrombus burden in patients with ST-segment elevation myocardial infarction. Biomarkers 24(6):600–606. https://doi.org/10.1080/1354750X.2019.1628809
Galli M et al (2018) 2018. Phase I study of the heparanase inhibitor roneparstat: an innovative approach for multiple myeloma therapy. Haematologica 103(10):e469–e472. https://doi.org/10.3324/haematol.2017.182865
Weissmann M et al (2019) The heparanase inhibitor PG545 is a potent anti-lymphoma drug: mode of action. Matrix Biol 77:58–72. https://doi.org/10.1016/j.matbio.2018.08.005
Masola V et al (2018) Inhibition of heparanase protects against chronic kidney dysfunction following ischemia/reperfusion injury. Oncotarget 9(90):36185–36201. https://doi.org/10.18632/oncotarget.26324
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. F. Yilmaz, E. Acar, M. Inanir, C. Y. Karabay and I. A. Izgi declare that they have no competing interests.
All procedures performed in studies involving human participants or on human tissue were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all participants included in the study.
Rights and permissions
About this article
Cite this article
Yilmaz, M.F., Acar, E., Inanir, M. et al. Serum heparanase levels and left atrial/left atrial appendage thrombus in patients with nonvalvular atrial fibrillation. Herz 47, 251–257 (2022). https://doi.org/10.1007/s00059-021-05052-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-021-05052-z