Abstract
Background
The importance of inflammation in thrombosis is increasingly appreciated. Neutrophil-lymphocyte ratio (NLR) and monocyte to high-density lipoprotein ratio (MHR) are important indicators of systemic inflammation. This study aimed to investigate the associations between NLR and MHR with left atrial appendage thrombus (LAAT) and spontaneous echo contrast (SEC) in patients with non-valvular atrial fibrillation.
Methods
This retrospective, cross-sectional study enrolled 569 consecutive patients with non-valvular atrial fibrillation. Multivariable logistic regression analysis was used to investigate independent risk factors of LAAT/SEC. Receiver operating characteristic (ROC) curves were used to evaluate the specificity and sensitivity of NLR and MHR in predicting LAAT/SEC. Subgroup and Pearson correlation analyses were used to assess the correlations between NLR and MHR with the CHA2DS2-VASc score.
Results
Multivariate logistic regression analysis showed that NLR (OR: 1.49; 95%CI: 1.173–1.892) and MHR (OR: 2.951; 95%CI: 1.045–8.336) were independent risk factors for LAAT/SEC. The area under the ROC curve of NLR (0.639) and MHR (0.626) was similar to that of the CHADS2 score (0.660) and CHA2DS2-VASc score (0.637). Subgroup and Pearson correlation analyses showed significant but very weak associations between NLR (r = 0.139, P < 0.05) and MHR (r = 0.095, P < 0.05) with the CHA2DS2-VASc score.
Conclusion
Generally, NLR and MHR are independent risk factors for predicting LAAT/SEC in patients with non-valvular atrial fibrillation.
Similar content being viewed by others
Introduction
Atrial fibrillation (AF) is the most common and clinically significant persistent cardiac arrhythmia, with a prevalence of 1–3% in the general population that increased with age [1, 2]. AF is associated with a fivefold increased risk for stroke, leading to high morbidity and mortality [3, 4]. Anticoagulant therapy has been recommended as the most efficacious way to reduce the risk of stroke among patients with AF by nearly 60%, according to a meta-analysis [5]. Thus, early identification of thrombosis and signs of risk is of paramount importance to initiate timely anticoagulant therapy for stroke prevention. The left atrium (LA) and left atrial appendage (LAA) are the primary sites of thrombosis, with over 90% of embolic strokes reported to be caused by left atrial appendage thrombosis (LAAT) [6]. Spontaneous echo contrast (SEC), defined as the echogenicity of blood in the absence of contrast agents, is another well-recognized precursor for thrombosis that indicates blood stasis [7]. The most sensitive method for the detection of LAAT and SEC is transesophageal echocardiography (TEE) [8, 9]. However, this invasive examination may increase the risk of complications such as esophageal trauma, causing an additional burden on the patients. Since LAAT and SEC are both preventable and treatable, a safer and non-invasive assessment method is needed to detect LAAT/SEC to guide early anticoagulant treatment and stroke prevention.
In previous studies, the CHADS2 and CHA2DS2-VASc scores are mostly used to assess the risk of stroke; however, recent studies also investigated their value in predicting LAAT/SEC [10]. Although widely recommended for the most of risks of stroke in AF patients, the above prediction models showed inconsistent performances in subsequent validation studies. For instance, A recent systematic review and meta-analysis suggested that the power of the CHA2DS2-VASc score in the prediction of stroke is modest, highlighting the need for models with higher accuracy [11]. Previous studies also showed only modest predictive performances of these conventional scoring systems for LAAT/SEC prediction [12, 13]. Another major limitation of the CHADS2 and CHA2DS2-VASc score systems is the lack of inclusion of other potential risk factors for stroke, such as cancer [14], arthritis [15], rheumatic disease [16], and chronic kidney disease [17], all of which indicate the presence of inflammation. Inflammation and thrombosis are closely connected processes, and growing evidence has shown that inflammation might play a critical role in the development of LAAT/SEC [18,19,20]. It is thus expected that some novel predictive biomarkers for inflammation may help refine the current stroke risk assessment system by providing a more accurate prediction of LAAT/SEC in patients with AF.
Neutrophil-lymphocyte ratio (NLR) and monocyte to high-density lipoprotein ratio (MHR) are inexpensive and easily obtained biomarkers for systemic inflammation, which leads to an increased risk of stroke and mortality [21, 22]. Beomseok et al. found that a high level of NLR is an independent risk factor for ischemic stroke in healthy individuals, indicating the possibility of reclassification for stroke incidence in patients with AF [23]. A recent study also suggested that NLR might be a beneficial predictor for the potential acute venous thromboembolism (VTE) [24]. It has been found that high-density lipoprotein cholesterol (HDL-C) could suppress the pro-inflammatory and pro-oxidant effects of monocytes [25] and that it also has anti-inflammatory, antioxidant, and anti-thrombotic effects [26]. Therefore, decreased HDL-C and increased monocytes, reflected as increased MHR, may be an indicator of inflammation. In recent years, NLR and MHR have been widely reported to be risk factors for cardiovascular diseases and to be associated with increased all-cause mortality [27,28,29]. Some studies also indicated that NLR and MHR might be associated with thromboembolic stroke in patients with non-valvular AF (NVAF) [30,31,32]. Although both NLR [33, 34] and MHR [35] have been well established to be predictive of new-onset AF and the risk of stroke, previous studies on inflammation biomarkers mostly focused on their associations with ischemic stroke in patients with AF rather than those at high risk but with no stroke. In addition, stroke risk stratification could be confounded by non-cardioembolic stroke, misleading the anticoagulant treatment for patients with AF. As a result, it might be more informative to establish the association between NLR/MHR and the more specific LAAT/SEC, instead of the general risk of stroke, to avoid confounding. To our knowledge, there has been no study exploring the value of NLR and MHR in the prediction of LAAT/SEC in patients with NVAF. Therefore, we conducted the current study to evaluate the associations between NLR and MHR with LAAT/SEC and their correlations with the CHA2DS2-VASc score.
Materials and methods
In this retrospective cross-sectional study, we aimed to investigate the associations between NLR and MHR with LAAT/SEC based on data available in the medical records of consecutive patients with NVAF who were admitted to the Xiamen Cardiovascular Hospital for radiofrequency catheter ablation from June 2019 to May 2021.
A total of 569 eligible patients diagnosed with NVAF who underwent TEE were enrolled in this study and assigned into two groups: the LAAT/SEC group with a diagnosis of LAAT or SEC as confirmed by TEE (n = 98), and a negative control group (n = 471). Patients with leukemia, recent allergy or infection, and liver dysfunction were excluded. Patients with heart diseases including valvular heart disease, acute myocardial infarction, rheumatic heart disease, and those on medications that might affect the complete blood count were also excluded. The study complied with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Xiamen Cardiovascular Hospital. All procedures in this study were performed in accordance with the institutional guidelines.
Demographic and clinical data of the patients were collected after admission. All the subjects were tested for complete blood count and lipid profile. NLR was calculated as the absolute neutrophil count divided by the absolute lymphocyte count, and MHR was calculated as the monocyte count divided by the level of HDL. LAAT/SEC was diagnosed through TEE performed by experienced sonographers. LAAT was defined as well-circumscribed, echogenic masses with a different texture but uniform consistency, as compared with the LA wall. SEC was defined as a dynamic smoke-like signal with a swirling pattern in the LA and LAA, which could be detected by excessive gain under appropriate gain settings [36]. The CHADS2 and CHA2DS2-VASc scores were calculated based on their respective indicator scores, and the patients were further divided into the following four subgroups based on their CHA2DS2-VASc scores: 0 (Group 1), 1 (Group 2), 2–4 (Group 3), and ≥ 5 (Group 4).
Statistical analysis
Statistical analyses were performed using the SPSS software (version 22.0). Normally distributed continuous data were presented as mean ± standard deviation (SD), and inter-group comparisons were performed using independent samples t-test or one-way ANOVA as appropriate. Possible risk factors of LAAT/SEC were analyzed using multivariate logistic regression analysis. Model 1 included all risk factors with P < 0.05 in univariate analysis, while Model 2 and Model 3 only included NLR, MHR, and the CHADS2 score or the CHA2DS2-VASc score. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the specificity and sensitivity of NLR and MHR for the prediction of LAAT/SEC. The cut-off score was selected as the point that maximized both sensitivity and specificity. Correlations of NLR and MHR with CHA2DS2-VASc scores were evaluated using the Pearson correlation coefficient (r) and visualized using a scatter plot. P < 0.05 indicated statistically significant differences.
Results
A total of 569 patients were included in this study, with 98 (17.2%) patients in the LAAT/SEC group and 471 (82.8%) in the control group. The clinical characteristics of the patients are summarized in Table 1. Significant differences between the LAAT/SEC group and the control group were found in NLR, MHR, CHADS2 score, and CHA2DS2-VASc score, as well as other clinical indicators such as white blood cell (WBC) count, neutrophil count, monocyte count, HDL, estimated glomerular filtration (eGFR), left atrial diameter (LAD), ejection fraction (EF), heart failure, previous stroke/transient ischemic attack (TIA), peripheral arterial disease, and chronic kidney disease (P < 0.05) (Table 1). As many of the patients were not on any anticoagulant drug before admission, the impact of anticoagulants could not be determined.
In multivariable logistic regression analysis (Model 1), all risk factors with P < 0.05 in univariate analysis were included, which showed that heart failure (OR: 3.876; 95%CI: 1.876–8.007), previous stroke/TIA (OR: 4.079; 95%CI: 1.417–11.743), LAD (OR: 1.148; 95%CI: 1.09–1.209), NLR (OR: 1.49; 95%CI: 1.173–1.892) and MHR (OR: 2.951; 95%CI: 1.045–8.336) were independent risk factors for LAAT/SEC (Table 2). However, neither the CHADS2 score nor the CHA2DS2-VASc score was statistically significantly associated with LAAT/SEC after controlling for other variables. Model 2 and Model 3 only included NLR, MHR, and the CHADS2 score or the CHA2DS2-VASc score, which showed that the CHADS2 score and CHA2DS2-VASc score were independent risk factors for LAAT/SEC after adjusting for NLR and MHR (Table 2).
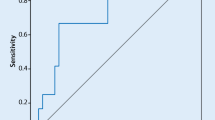
The ROC curve analyses of NLR, MHR, CHADS2 score and CHA2DS2-VASc score in the prediction of LAAT/SEC are presented in Fig. 1; Table 3. All four indicators demonstrated comparable discrimination ability in distinguishing the LAAT/SEC group from the control group. The area under the ROC curve (AUC) of NLR (AUC = 0.639) and MHR (AUC = 0.626) were similar to that of CHADS2 score (AUC = 0.660) and CHA2DS2-VASc score (AUC = 0.637). As for sensitivity and specificity in predicting LAAT/SEC, an NLR cut-off score of 2.57 showed a sensitivity of 0.561 and specificity of 0.686, while an MHR cut-off score of 0.566 showed a sensitivity of 0.347 and specificity of 0.873 (Table 3). Across the four indicators, the CHA2DS2-VASc score showed the highest sensitivity (0.786), and MHR showed the highest specificity (0.873) in the ROC curve analysis (Table 3).
The results of subgroup analyses on the prevalence of LAAT/SEC and levels of NLR and MHR according to the CHA2DS2-VASc score classification are presented in Table 4. Generally, there was an increasing trend of LAAT/SEC, NLR, and MHR with the CHA2DS2-VASc score. In the comparison between subgroups, the incidence of LAAT/SEC was significantly higher in group 3 (19.66%) and group 4 (34.48%), as compared with group 1 (8.79%) and group 2 (10.00%). The NLR was also significantly higher in group 3 (2.55 ± 1.15) and group 4 (2.69 ± 1.41), as compared with group 1 (2.27 ± 0.91) and group 2 (2.20 ± 0.84). The MHR was significantly higher in group 4 (0.55 ± 0.67) only, as compared with group 1 (0.42 ± 0.19), group 2 (0.41 ± 0.26), and group 3 (0.42 ± 0.19) (Table 4).
The correlations of NLR and MHR with the CHA2DS2-VASc score in patients with NVAF are presented in Fig. 2. Both NLR and MHR showed significant correlations with the CHA2DS2-VASc score, but the correlation coefficients were small (r = 0.139 and P < 0.05 for NLR; r = 0.095 and P < 0.05 for MHR).
Discussion
In this cross-sectional study, we innovatively used two inflammation indicators, i.e., NLR and MHR, to investigate their associations with LAAT/SEC in patients with NVAF and evaluated their predictive performances as compared to the conventional CHADS2 and CHA2DS2-VASc score systems. Our major findings showed that both NLR and MHR are independent risk factors for predicting LAAT/SEC in patients with NVAF. Further subgroup and Pearson correlation analyses showed significant but very weak associations between NLR and MHR with the CHA2DS2-VASc score, suggesting their implication for the reclassification improvement of ischemic stroke in patients with NVAF.
Our major finding was that NLR and MHR were comparable and relatively independent from the conventional CHADS2 and CHA2DS2-VASc score systems in predicting LAAT/SEC among NVAF patients. NLR and MHR reflect the balance of neutrophils, lymphocytes, monocytes, and high-density lipoprotein in inflammatory and immune responses. The possible mechanism of NLR and MHR affecting thrombosis may include the following: (1) neutrophil extracellular traps (NETs) released by neutrophils play a critical role in the mechanisms underlying thrombosis, as demonstrated by recent studies [37,38,39]; (2) the majority of tissue factors associated with thrombosis are derived from monocytes [40], which also regulate the resolution of thrombus, with different monocyte subtypes playing different roles [41]; (3) lymphocytes have also been demonstrated to regulate the composition of thrombosis [42, 43]; and (4) HDL-C has anti-inflammatory, antioxidant, and anti-thrombotic effects, which may explain the underlying mechanisms on thrombosis, while low-density lipoprotein has been shown to promote thrombosis [44].
The associations between NLR and MHR with LAAT/SEC were consistent with previous evidence showing that inflammation was a contributing factor to AF and stroke [45,46,47,48]. Although it has been recognized that an inflammatory state can lead to coagulation, the underlying mechanism remains unclear. One possible explanation is that inflammation leads to a higher risk of thrombosis through upregulating procoagulant factors and downregulating anticoagulant factors and fibrinolytic activities [49]. In addition, inflammatory mediators may also increase platelet reactivities, leading to a prothrombotic state and thereby promoting thrombosis [50, 51]. In 2013, Engelmann et al. first used the term “immunothrombosis” to describe a physiological type of thrombosis in microvessels induced by immune cells and thrombosis-specific molecular mediators [52]. According to Engelmann et al., immunothrombosis involves a platform consisting of fibrin, neutrophils, monocytes, and platelets, which is also similar to large venous thrombosis [52]. On this basis, inflammation seems to be an important participant, rather than a bystander, in the process of thrombosis that leads to stroke. Although the CHADS2 or CHA2DS2-VASc score showed a significant association with LAAT/SEC in the univariate analysis, this association was insignificant in the subsequent multivariate regression when controlling for NLR, MHR, and other clinical factors. Further subgroup and Pearson correlation analyses showed very weak associations between NLR and MHR with CHA2DS2-VASc. These findings have further indicated the comparable and supplementary utility of NLR and MHR in stroke risk assessment above the conventional CHADS2 and CHA2DS2-VASc score systems.
Subgroup analyses based on the CHA2DS2-VASc score were performed to investigate the correlations between NLR and MHR and this conventional score model. The results showed that the incidence of LAAT/SEC and the levels of NLR and MHR were significantly higher in patients with higher CHA2DS2-VASc scores (≥ 2), which was consistent with findings in previous studies. Similarly, Gokhan et al. found that the CHADS2 score was significantly higher in the group with high NLR levels, suggesting that NLR might be a predictor of thromboembolic stroke in patients with NVAF [32]. Kahraman et al. suggested that NLR was related to the CHA2DS2-VASc score and was predictive of the risk of thromboembolism and hemorrhage [53]. However, to what extent NLR and MHR are related to the CHA2DS2-VASc score still remains unknown. Although both NLR and MHR showed significant correlations with the CHA2DS2-VASc score, the correlations were very weak, suggesting the possibility of relative independence of NLR and MHR from the CHA2DS2-VASc score. This result suggested that NLR and MHR, easily accessible clinical parameters, might further assist physicians to identify patients at high risk for stroke.
Conclusion
In this study, we found that NLR and MHR were independent risk factors for predicting LAAT/SEC in patients with NVAF. NLR and MHR had comparable performances with the conventional CHADS2 score and CHA2DS2-VASc score in predicting LAAT/SEC while making up for the limitations of these conventional score systems. NLR and MHR may be used as alternative stroke risk stratification schemes in clinical practice.
Data Availability
The datasets used during the current study are available from the corresponding author on reasonable request.
References
Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and risk factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–5.
Freedman B, Hindricks G, Banerjee A, Baranchuk A, Ching CK, Du X, Fitzsimons D, Healey JS, Ikeda T, Lobban TCA, et al. World Heart Federation Roadmap on Atrial Fibrillation - A 2020 Update. Glob Heart. 2021;16(1):41.
Gomez-Outes A, Lagunar-Ruiz J, Terleira-Fernandez AI, Calvo-Rojas G, Suarez-Gea ML, Vargas-Castrillon E. Causes of death in anticoagulated patients with Atrial Fibrillation. J Am Coll Cardiol. 2016;68(23):2508–21.
Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–8.
Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857–67.
Beigel R, Wunderlich NC, Ho SY, Arsanjani R, Siegel RJ. The left atrial appendage: anatomy, function, and noninvasive evaluation. JACC Cardiovasc Imaging. 2014;7(12):1251–65.
Erbel R, Stern H, Ehrenthal W, Schreiner G, Treese N, Kramer G, Thelen M, Schweizer P, Meyer J. Detection of spontaneous echocardiographic contrast within the left atrium by transesophageal echocardiography: spontaneous echocardiographic contrast. Clin Cardiol. 1986;9(6):245–52.
Romero J, Cao JJ, Garcia MJ, Taub CC. Cardiac imaging for assessment of left atrial appendage stasis and thrombosis. Nat Rev Cardiol. 2014;11(8):470–80.
Ellis K, Ziada KM, Vivekananthan D, Latif AA, Shaaraoui M, Martin D, Grimm RA. Transthoracic echocardiographic predictors of left atrial appendage thrombus. Am J Cardiol. 2006;97(3):421–5.
Zhan Y, Joza J, Al Rawahi M, Barbosa RS, Samuel M, Bernier M, Huynh T, Thanassoulis G, Essebag V. Assessment and Management of the left atrial appendage Thrombus in patients with Nonvalvular Atrial Fibrillation. Can J Cardiol. 2018;34(3):252–61.
Siddiqi TJ, Usman MS, Shahid I, Ahmed J, Khan SU, Ya’qoub L, Rihal CS, Alkhouli M. Utility of the CHA2DS2-VASc score for predicting ischaemic stroke in patients with or without atrial fibrillation: a systematic review and meta-analysis. Eur J Prev Cardiol. 2022;29(4):625–31.
Huang J, Wu SL, Xue YM, Fei HW, Lin QW, Ren SQ, Liao HT, Zhan XZ, Fang XH, Xu L. Association of CHADS2 and CHA2DS2-VASc scores with left atrial Thrombus with Nonvalvular Atrial Fibrillation: a single Center based Retrospective Study in a cohort of 2695 chinese subjects. Biomed Res Int. 2017;2017:6839589.
Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–72.
Grazioli S, Paciaroni M, Agnelli G, Acciarresi M, Alberti A, D’Amore C, Caso V, Venti M, Guasti L, Ageno W, et al. Cancer-associated ischemic stroke: a retrospective multicentre cohort study. Thromb Res. 2018;165:33–7.
Liu W, Ma W, Liu H, Li C, Zhang Y, Liu J, Liang Y, Zhang S, Wu Z, Zang C, et al. Stroke risk in arthritis: a systematic review and meta-analysis of cohort studies. PLoS ONE. 2021;16(3):e0248564.
Wiseman SJ, Ralston SH, Wardlaw JM. Cerebrovascular Disease in Rheumatic Diseases: a systematic review and Meta-analysis. Stroke. 2016;47(4):943–50.
Olesen JB, Lip GY, Kamper AL, Hommel K, Kober L, Lane DA, Lindhardsen J, Gislason GH, Torp-Pedersen C. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012;367(7):625–35.
Maehama T, Okura H, Imai K, Saito K, Yamada R, Koyama T, Hayashida A, Neishi Y, Kawamoto T, Yoshida K. Systemic inflammation and left atrial thrombus in patients with non-rheumatic atrial fibrillation. J Cardiol. 2010;56(1):118–24.
Makita S, Nakamura M, Satoh K, Tanaka F, Onoda T, Kawamura K, Ohsawa M, Tanno K, Itai K, Sakata K, et al. Serum C-reactive protein levels can be used to predict future ischemic stroke and mortality in japanese men from the general population. Atherosclerosis. 2009;204(1):234–8.
Stark K, Massberg S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat Rev Cardiol. 2021;18(9):666–82.
Katipoglu Z, Mirza E, Oltulu R, Katipoglu B. May Monocyte/HDL cholesterol ratio (MHR) and Neutrophil/Lymphocyte ratio (NLR) be an Indicator of inflammation and oxidative stress in patients with Keratoconus? Ocul Immunol Inflamm. 2020;28(4):632–6.
Aktas Karabay E, Demir D, Aksu Cerman A. Evaluation of monocyte to high-density lipoprotein ratio, lymphocytes, monocytes, and platelets in psoriasis. An Bras Dermatol. 2020;95(1):40–5.
Suh B, Shin DW, Kwon HM, Yun JM, Yang HK, Ahn E, Lee H, Park JH, Cho B. Elevated neutrophil to lymphocyte ratio and ischemic stroke risk in generally healthy adults. PLoS ONE. 2017;12(8):e0183706.
Farah R, Nseir W, Kagansky D, Khamisy-Farah R. The role of neutrophil-lymphocyte ratio, and mean platelet volume in detecting patients with acute venous thromboembolism. J Clin Lab Anal. 2020;34(1):e23010.
Westerterp M, Gourion-Arsiquaud S, Murphy AJ, Shih A, Cremers S, Levine RL, Tall AR, Yvan-Charvet L. Regulation of hematopoietic stem and progenitor cell mobilization by cholesterol efflux pathways. Cell Stem Cell. 2012;11(2):195–206.
Diakowska D, Grabowski K, Nienartowicz M, Zarebski P, Fudalej K, Markocka-Maczka K. Circulating oxidized low-density lipoproteins and antibodies against oxidized low-density lipoproteins as potential biomarkers of Colorectal Cancer. Gastroenterol Res Pract. 2015;2015:146819.
Adamstein NH, MacFadyen JG, Rose LM, Glynn RJ, Dey AK, Libby P, Tabas IA, Mehta NN, Ridker PM. The neutrophil-lymphocyte ratio and incident atherosclerotic events: analyses from five contemporary randomized trials. Eur Heart J. 2021;42(9):896–903.
Sun M, Zhao D, Zhang Y, Zhai Y, Ye M, Wang X, Zheng L, Wang L. Prognostic utility of monocyte to high-density lipoprotein ratio in patients with Acute Coronary Syndrome: a Meta-analysis. Am J Med Sci. 2020;359(5):281–6.
Horne BD, Anderson JL, John JM, Weaver A, Bair TL, Jensen KR, Renlund DG, Muhlestein JB. Intermountain Heart Collaborative Study G: which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005;45(10):1638–43.
Liu H, Liu K, Pei L, Gao Y, Zhao L, Sun S, Wu J, Li Y, Fang H, Song B, et al. Monocyte-to-high-density lipoprotein ratio predicts the outcome of Acute ischemic stroke. J Atheroscler Thromb. 2020;27(9):959–68.
Liu H, Zhan F, Wang Y. Evaluation of monocyte-to-high-density lipoprotein cholesterol ratio and monocyte-to-lymphocyte ratio in ischemic stroke. J Int Med Res. 2020;48(7):300060520933806.
Ertas G, Sonmez O, Turfan M, Kul S, Erdogan E, Tasal A, Bacaksiz A, Vatankulu MA, Altintas O, Uyarel H, et al. Neutrophil/lymphocyte ratio is associated with thromboembolic stroke in patients with non-valvular atrial fibrillation. J Neurol Sci. 2013;324(1–2):49–52.
Berkovitch A, Younis A, Grossman Y, Segev S, Kivity S, Sidi Y, Beinart R, Goldenberg I, Maor E. Relation of neutrophil to lymphocyte ratio to risk of Incident Atrial Fibrillation. Am J Cardiol. 2019;123(3):396–401.
Baş HA, BAĞCI A, Aksoy F. Usefulness of mean platelet volume and neutrophil-to-lymphocyte ratio for development of atrial fibrillation after acute myocardial infarction. Süleyman Demirel Üniversitesi Sağlık Bilimleri Dergisi, 10(3):278–83.
Ulus T, Isgandarov K, Yilmaz AS, Vasi I, Moghanchizadeh SH, Mutlu F. Predictors of new-onset atrial fibrillation in elderly patients with acute coronary syndrome undergoing percutaneous coronary intervention. Aging Clin Exp Res. 2018;30(12):1475–82.
Masuda M, Iwakura K, Inoue K, Okamura A, Koyama Y, Toyoshima Y, Tanaka N, Nakanishi H, Sotomi Y, Komuro I, et al. Estimation of left atrial blood stasis using diastolic late mitral annular velocity. Eur Heart J Cardiovasc Imaging. 2013;14(8):752–7.
Chen X, Wang L, Jiang M, Lin L, Ba Z, Tian H, Li G, Chen L, Liu Q, Hou X, et al. Leukocytes in cerebral Thrombus respond to large-vessel occlusion in a time-dependent Manner and the Association of NETs with collateral Flow. Front Immunol. 2022;13:834562.
Carminita E, Crescence L, Panicot-Dubois L, Dubois C. Role of Neutrophils and NETs in Animal Models of Thrombosis. Int J Mol Sci 2022, 23(3).
Behzadifard M, Soleimani M. NETosis and SARS-COV-2 infection related thrombosis: a narrative review. Thromb J. 2022;20(1):13.
Swystun LL, Liaw PC. The role of leukocytes in thrombosis. Blood. 2016;128(6):753–62.
Shahneh F, Christian Probst H, Wiesmann SC, Ruf NAG, Steinbrink W, Raker K, Becker VK. Inflammatory monocyte counts determine venous blood clot formation and resolution. Arterioscler Thromb Vasc Biol. 2022;42(2):145–55.
Bennett JA, Mastrangelo MA, Ture SK, Smith CO, Loelius SG, Berg RA, Shi X, Burke RM, Spinelli SL, Cameron SJ, et al. The choline transporter Slc44a2 controls platelet activation and thrombosis by regulating mitochondrial function. Nat Commun. 2020;11(1):3479.
Luther N, Shahneh F, Brahler M, Krebs F, Jackel S, Subramaniam S, Stanger C, Schonfelder T, Kleis-Fischer B, Reinhardt C, et al. Innate effector-memory T-Cell activation regulates post-thrombotic vein wall inflammation and Thrombus resolution. Circ Res. 2016;119(12):1286–95.
Ding WY, Protty MB, Davies IG, Lip GYH. Relationship between lipoproteins, thrombosis, and atrial fibrillation. Cardiovasc Res. 2022;118(3):716–31.
Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60(22):2263–70.
Anrather J, Iadecola C. Inflammation and stroke: an overview. Neurotherapeutics. 2016;13(4):661–70.
Harada M, Van Wagoner DR, Nattel S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ J. 2015;79(3):495–502.
Bagci A, Aksoy F. Systemic immune-inflammation index predicts new-onset atrial fibrillation after ST elevation myocardial infarction. Biomark Med. 2021;15(10):731–9.
Aksu K, Donmez A, Keser G. Inflammation-induced thrombosis: mechanisms, disease associations and management. Curr Pharm Des. 2012;18(11):1478–93.
Esmon CT. Inflammation and thrombosis. J Thromb Haemost. 2003;1(7):1343–8.
Libby P, Simon DI. Inflammation and thrombosis: the clot thickens. Circulation. 2001;103(13):1718–20.
Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13(1):34–45.
Cosansu K, Vatan MB, Gunduz H, Akdemir R. Use of neutrophil-lymphocyte ratio for risk stratification and relationship with time in therapeutic range in patients with nonvalvular atrial fibrillation: a pilot study. Clin Cardiol. 2018;41(3):339–42.
Acknowledgements
We thank all participants and all investigators.
Funding
This research was supported by the Scientific and technological innovation joint capital projects of Fujian Province (2020Y2016), the Fujian Provincial Health and Family Planning Youth Scientific Research Project (2022QNB029), and the Medical and health care guideline project of Xiamen(No.3502Z20214ZD1280).
Author information
Authors and Affiliations
Contributions
YJD, QZ, and QL designed the study; YJD, QZ, QL, LLL, JHL, and GYL collected and analyzed the data; YJD drafted the manuscript; FGZ, JCG, and BNC provided feedback on earlier drafts of the manuscript; QZ and DC reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study complied with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Xiamen Cardiovascular Hospital. Informed consent was obtained from all subjects and/or their legal guardian(s).
Consent for publication
Not applicable.
Conflict of Interest
The authors declare that they have no potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Deng, Y., Zhou, F., Li, Q. et al. Associations between neutrophil-lymphocyte ratio and monocyte to high-density lipoprotein ratio with left atrial spontaneous echo contrast or thrombus in patients with non-valvular atrial fibrillation. BMC Cardiovasc Disord 23, 234 (2023). https://doi.org/10.1186/s12872-023-03270-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-023-03270-3