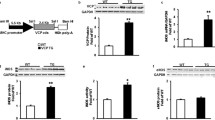
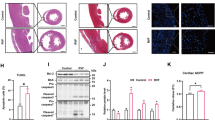
Abstract
Dual specificity phosphatase 1 (DUSP1) and valosin-containing protein (VCP) have both been reported to regulate mitochondrial homeostasis. However, their impact on mitochondrial quality control (MQC) and myocardial function during LPS-induced endotoxemia remains unclear. We addressed this issue by modeling LPS-induced endotoxemia in DUSP1 transgenic (DUSP1TG) mice and in cultured DUSP1-overexpressing HL-1 cardiomyocytes. Accompanying characteristic structural and functional deficits, cardiac DUSP1 expression was significantly downregulated following endotoxemia induction in wild type mice. In contrast, markedly reduced myocardial inflammation, cardiomyocyte apoptosis, cardiac structural disorder, cardiac injury marker levels, and normalized systolic/diastolic function were observed in DUSP1TG mice. Furthermore, DUSP1 overexpression in HL-1 cells significantly attenuated LPS-mediated mitochondrial dysfunction by preserving MQC, as indicated by normalized mitochondrial dynamics, improved mitophagy, enhanced biogenesis, and attenuated mitochondrial unfolded protein response. Molecular assays showed that VCP was a substrate of DUSP1 and the interaction between DUSP1 and VCP primarily occurred on the mitochondria. Mechanistically, DUSP1 phosphatase domain promoted the physiological DUSP1/VCP interaction which prevented LPS-mediated VCP Ser784 phosphorylation. Accordingly, transfection with a phosphomimetic VCP mutant abolished the protective actions of DUSP1 on MQC and aggravated inflammation, apoptosis, and contractility/relaxation capacity in HL-1 cardiomyocytes. These findings support the involvement of the novel DUSP1/VCP/MQC pathway in the pathogenesis of endotoxemia-caused myocardial dysfunction.









Similar content being viewed by others
Data availability
The data supporting the findings of this study are found within the article and the supplementary material. All relevant raw data will be made available from the corresponding author upon reasonable request.
References
Vieillard-Baron A (2011) Septic cardiomyopathy. Ann Intensive Care 1:6
Martin L, Derwall M, Al Zoubi S, Zechendorf E, Reuter DA, Thiemermann C, Schuerholz T (2019) The septic heart: current understanding of molecular mechanisms and clinical implications. Chest 155:427–437
Tan Y, Chen S, Zhong J, Ren J, Dong M (2019) Mitochondrial injury and targeted intervention in septic cardiomyopathy. Curr Pharm Des 25:2060–2070
Cimolai MC, Alvarez S, Bode C, Bugger H (2015) Mitochondrial mechanisms in septic cardiomyopathy. Int J Mol Sci 16:17763–17778
Shang X, Zhang Y, Xu J, Li M, Wang X, Yu R (2020) SRV2 promotes mitochondrial fission and Mst1-Drp1 signaling in LPS-induced septic cardiomyopathy. Aging (Albany NY) 12:1417–1432
Durand A, Duburcq T, Dekeyser T, Neviere R, Howsam M, Favory R, Preau S (2017) Involvement of mitochondrial disorders in septic cardiomyopathy. Oxid Med Cell Longev 2017:4076348
Ji L, He Q, Liu Y, Deng Y, Xie M, Luo K, Cai X, Zuo Y, Wu W, Li Q, Zhou R, Li T (2022) Ketone body β-hydroxybutyrate prevents myocardial oxidative stress in septic cardiomyopathy. Oxid Med Cell Longev 2022:2513837
Zhong J, Tan Y, Lu J, Liu J, Xiao X, Zhu P, Chen S, Zheng S, Chen Y, Hu Y, Guo Z (2019) Therapeutic contribution of melatonin to the treatment of septic cardiomyopathy: a novel mechanism linking Ripk3-modified mitochondrial performance and endoplasmic reticulum function. Redox Biol 26:101287
Jiang X, Cai S, Jin Y, Wu F, He J, Wu X, Tan Y, Wang Y (2021) Irisin attenuates oxidative stress, mitochondrial dysfunction, and apoptosis in the H9C2 cellular model of septic cardiomyopathy through augmenting Fundc1-dependent mitophagy. Oxid Med Cell Longev 2021:2989974
Wang Y, Jasper H, Toan S, Muid D, Chang X, Zhou H (2021) Mitophagy coordinates the mitochondrial unfolded protein response to attenuate inflammation-mediated myocardial injury. Redox Biol 45:102049
Teng CH, Huang WN, Meng TC (2007) Several dual specificity phosphatases coordinate to control the magnitude and duration of JNK activation in signaling response to oxidative stress. J Biol Chem 282:28395–28407
Shen J, Zhang Y, Yu H, Shen B, Liang Y, Jin R, Liu X, Shi L, Cai X (2016) Role of DUSP1/MKP1 in tumorigenesis, tumor progression and therapy. Cancer Med 5:2061–2068
Keyse SM (2008) Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev 27:253–261
Jin Q, Li R, Hu N, Xin T, Zhu P, Hu S, Ma S, Zhu H, Ren J, Zhou H (2018) DUSP1 alleviates cardiac ischemia/reperfusion injury by suppressing the Mff-required mitochondrial fission and Bnip3-related mitophagy via the JNK pathways. Redox Biol 14:576–587
Bermúdez-Muñoz JM, Celaya AM, García-Mato Á, Muñoz-Espín D, Rodríguez-De La Rosa L, Serrano M, Varela-Nieto I (2021) Dual-specificity phosphatase 1 (DUSP1) has a central role in redox homeostasis and inflammation in the mouse cochlea. Antioxidants (Basel) 10:1351
Sheng J, Li H, Dai Q, Lu C, Xu M, Zhang J, Feng J (2019) DUSP1 recuses diabetic nephropathy via repressing JNK-Mff-mitochondrial fission pathways. J Cell Physiol 234:3043–3057
Hou X, Li L, Chen S, Ge C, Shen M, Fu Z (2021) MKP-1 overexpression reduces postischemic myocardial damage through attenuation of ER stress and mitochondrial damage. Oxid Med Cell Longev 2021:8905578
Huang D, Jiang Y (2020) MKP1 reduces neuroinflammation via inhibiting endoplasmic reticulum stress and mitochondrial dysfunction. J Cell Physiol 235:4316–4325
Lawan A, Min K, Zhang L, Canfran-Duque A, Jurczak MJ, Camporez JPG, Nie Y, Gavin TP, Shulman GI, Fernandez-Hernando C, Bennett AM (2018) Skeletal muscle-specific deletion of MKP-1 reveals a p38 MAPK/JNK/Akt signaling node that regulates obesity-induced insulin resistance. Diabetes 67:624–635
Ding YH, Miao RX, Zhang Q (2021) Hypaphorine exerts anti-inflammatory effects in sepsis induced acute lung injury via modulating DUSP1/p38/JNK pathway. Kaohsiung J Med Sci 37:883–893
Hammer M, Mages J, Dietrich H, Servatius A, Howells N, Cato AC, Lang R (2006) Dual specificity phosphatase 1 (DUSP1) regulates a subset of LPS-induced genes and protects mice from lethal endotoxin shock. J Exp Med 203:15–20
Zhang J, Xu X, Wang M (2021) Clinical significance of serum miR-101-3p expression in patients with neonatal sepsis. Per Med 18:541–550
Brudecki L, Ferguson DA, Mccall CE, El Gazzar M (2013) Mitogen-activated protein kinase phosphatase 1 disrupts proinflammatory protein synthesis in endotoxin-adapted monocytes. Clin Vaccine Immunol 20:1396–1404
Frazier WJ, Wang X, Wancket LM, Li XA, Meng X, Nelin LD, Cato AC, Liu Y (2009) Increased inflammation, impaired bacterial clearance, and metabolic disruption after gram-negative sepsis in Mkp-1-deficient mice. J Immunol 183:7411–7419
Watts GD, Wymer J, Kovach MJ, Mehta SG, Mumm S, Darvish D, Pestronk A, Whyte MP, Kimonis VE (2004) Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet 36:377–381
Nalbandian A, Llewellyn KJ, Gomez A, Walker N, Su H, Dunnigan A, Chwa M, Vesa J, Kenney MC, Kimonis VE (2015) In vitro studies in VCP-associated multisystem proteinopathy suggest altered mitochondrial bioenergetics. Mitochondrion 22:1–8
Ide Y, Horie T, Saito N, Watanabe S, Otani C, Miyasaka Y, Kuwabara Y, Nishino T, Nakao T, Nishiga M, Nishi H, Nakashima Y, Nakazeki F, Koyama S, Kimura M, Tsuji S, Rodriguez RR, Xu S, Yamasaki T, Watanabe T, Yamamoto M, Yanagita M, Kimura T, Kakizuka A, Ono K (2019) Cardioprotective effects of VCP modulator KUS121 in murine and porcine models of myocardial infarction. JACC Basic Transl Sci 4:701–714
Guo X, Sun X, Hu D, Wang YJ, Fujioka H, Vyas R, Chakrapani S, Joshi AU, Luo Y, Mochly-Rosen D, Qi X (2016) VCP recruitment to mitochondria causes mitophagy impairment and neurodegeneration in models of Huntington’s disease. Nat Commun 7:12646
Ogor P, Yoshida T, Koike M, Kakizuka A (2021) VCP relocalization limits mitochondrial activity, GSH depletion and ferroptosis during starvation in PC3 prostate cancer cells. Genes Cells 26:570–582
Kim NC, Tresse E, Kolaitis RM, Molliex A, Thomas RE, Alami NH, Wang B, Joshi A, Smith RB, Ritson GP, Winborn BJ, Moore J, Lee JY, Yao TP, Pallanck L, Kundu M, Taylor JP (2013) VCP is essential for mitochondrial quality control by PINK1/Parkin and this function is impaired by VCP mutations. Neuron 78:65–80
Yu CC, Yang JC, Chang YC, Chuang JG, Lin CW, Wu MS, Chow LP (2013) VCP phosphorylation-dependent interaction partners prevent apoptosis in Helicobacter pylori-infected gastric epithelial cells. PLoS ONE 8:e55724
Shao J (2020) Ser(784) phosphorylation: a clinically relevant enhancer of VCP function in the DNA damage response. Mol Cell Oncol 7:1796179
Zhu C, Rogers A, Asleh K, Won J, Gao D, Leung S, Li S, Vij KR, Zhu J, Held JM, You Z, Nielsen TO, Shao J (2020) Phospho-Ser(784)-VCP is required for dna damage response and is associated with poor prognosis of chemotherapy-treated breast cancer. Cell Rep 31:107745
Sun X, Zhou N, Ma B, Wu W, Stoll S, Lai L, Qin G, Qiu H (2021) Functional inhibition of valosin-containing protein induces cardiac dilation and dysfunction in a new dominant-negative transgenic mouse model. Cells 10:2891
Brody MJ, Vanhoutte D, Bakshi CV, Liu R, Correll RN, Sargent MA, Molkentin JD (2019) Disruption of valosin-containing protein activity causes cardiomyopathy and reveals pleiotropic functions in cardiac homeostasis. J Biol Chem 294:8918–8929
Zhou N, Chen X, Xi J, Ma B, Leimena C, Stoll S, Qin G, Wang C, Qiu H (2020) Novel genomic targets of valosin-containing protein in protecting pathological cardiac hypertrophy. Sci Rep 10:18098
Lizano P, Rashed E, Stoll S, Zhou N, Wen H, Hays TT, Qin G, Xie LH, Depre C, Qiu H (2017) The valosin-containing protein is a novel mediator of mitochondrial respiration and cell survival in the heart in vivo. Sci Rep 7:46324
Zhou N, Stoll S, Qiu H (2017) VCP represses pathological cardiac hypertrophy. Aging (Albany NY) 9:2469–2470
Kho DH, Uddin MH, Chatterjee M, Vogt A, Raz A, Wu GS (2019) GP78 cooperates with dual-specificity phosphatase 1 to stimulate epidermal growth factor receptor-mediated extracellular signal-regulated kinase signaling. Mol Cell Biol 39:e00485-18
Li Y, Xu S, Xu Q, Chen Y (2020) Clostridium difficile toxin B induces colonic inflammation through the TRIM46/DUSP1/MAPKs and NF-κB signalling pathway. Artif Cells Nanomed Biotechnol 48:452–462
Hammer M, Echtenachter B, Weighardt H, Jozefowski K, Rose-John S, Männel DN, Holzmann B, Lang R (2010) Increased inflammation and lethality of Dusp1-/- mice in polymicrobial peritonitis models. Immunology 131:395–404
Rodriguez N, Dietrich H, Mossbrugger I, Weintz G, Scheller J, Hammer M, Quintanilla-Martinez L, Rose-John S, Miethke T, Lang R (2010) Increased inflammation and impaired resistance to Chlamydophila pneumoniae infection in Dusp1(-/-) mice: critical role of IL-6. J Leukoc Biol 88:579–587
Chang W, Feng M, Li Y, Sun Y, Sun L (2019) MKP1 overexpression reduces TNF-α-induced cardiac injury via suppressing mitochondrial fragmentation and inhibiting the JNK-MIEF1 pathways. J Cell Physiol. https://doi.org/10.1002/jcp.28273
Cooper LT, Hare JM, Tazelaar HD, Edwards WD, Starling RC, Deng MC, Menon S, Mullen GM, Jaski B, Bailey KR, Cunningham MW, Dec GW (2008) Usefulness of immunosuppression for giant cell myocarditis. Am J Cardiol 102:1535–1539
Frustaci A, Russo MA, Chimenti C (2009) Randomized study on the efficacy of immunosuppressive therapy in patients with virus-negative inflammatory cardiomyopathy: the TIMIC study. Eur Heart J 30:1995–2002
Wojnicz R, Nowalany-Kozielska E, Wojciechowska C, Glanowska G, Wilczewski P, Niklewski T, Zembala M, Polonski L, Rozek MM, Wodniecki J (2001) Randomized, placebo-controlled study for immunosuppressive treatment of inflammatory dilated cardiomyopathy: two-year follow-up results. Circulation 104:39–45
Kwon HJ, Coté TR, Cuffe MS, Kramer JM, Braun MM (2003) Case reports of heart failure after therapy with a tumor necrosis factor antagonist. Ann Intern Med 138:807–811
Mann DL, Mcmurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, Djian J, Drexler H, Feldman A, Kober L, Krum H, Liu P, Nieminen M, Tavazzi L, Van Veldhuisen DJ, Waldenstrom A, Warren M, Westheim A, Zannad F, Fleming T (2004) Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation 109:1594–1602
Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT (2003) Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 107:3133–3140
Wang J, Zhou H (2020) Mitochondrial quality control mechanisms as molecular targets in cardiac ischemia-reperfusion injury. Acta Pharm Sin B 10:1866–1879
Zhou H, Ren J, Toan S, Mui D (2021) Role of mitochondrial quality surveillance in myocardial infarction: from bench to bedside. Ageing Res Rev 66:101250
Picca A, Mankowski RT, Burman JL, Donisi L, Kim JS, Marzetti E, Leeuwenburgh C (2018) Mitochondrial quality control mechanisms as molecular targets in cardiac ageing. Nat Rev Cardiol 15:543–554
Ikeda Y, Shirakabe A, Maejima Y, Zhai P, Sciarretta S, Toli J, Nomura M, Mihara K, Egashira K, Ohishi M, Abdellatif M, Sadoshima J (2015) Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ Res 116:264–278
Qiu Z, Wei Y, Song Q, Du B, Wang H, Chu Y, Hu Y (2019) The role of myocardial mitochondrial quality control in heart failure. Front Pharmacol 10:1404
Shirakabe A, Zhai P, Ikeda Y, Saito T, Maejima Y, Hsu CP, Nomura M, Egashira K, Levine B, Sadoshima J (2016) Drp1-dependent mitochondrial autophagy plays a protective role against pressure overload-induced mitochondrial dysfunction and heart failure. Circulation 133:1249–1263
Yu LM, Dong X, Xue XD, Xu S, Zhang X, Xu YL, Wang ZS, Wang Y, Gao H, Liang YX, Yang Y, Wang HS (2021) Melatonin attenuates diabetic cardiomyopathy and reduces myocardial vulnerability to ischemia-reperfusion injury by improving mitochondrial quality control: role of SIRT6. J Pineal Res 70:e12698
Viswanathan MC, Blice-Baum AC, Sang TK, Cammarato A (2016) Cardiac-restricted expression of VCP/TER94 RNAi or disease alleles perturbs drosophila heart structure and impairs function. J Cardiovasc Dev Dis 3:19
Yi P, Higa A, Taouji S, Bexiga MG, Marza E, Arma D, Castain C, Le Bail B, Simpson JC, Rosenbaum J, Balabaud C, Bioulac-Sage P, Blanc JF, Chevet E (2012) Sorafenib-mediated targeting of the AAA+ ATPase p97/VCP leads to disruption of the secretory pathway, endoplasmic reticulum stress, and hepatocellular cancer cell death. Mol Cancer Ther 11:2610–2620
Zhu K, Cai Y, Si X, Ye Z, Gao Y, Liu C, Wang R, Ma Z, Zhu H, Zhang L, Li S, Zhang H, Yue J (2022) The phosphorylation and dephosphorylation switch of VCP/p97 regulates the architecture of centrosome and spindle. Cell Death Differ. https://doi.org/10.1038/s41418-022-01000-4
Zou R, Tao J, Qiu J, Lu H, Wu J, Zhu H, Li R, Mui D, Toan S, Chang X, Zhou H, Fan X (2022) DNA-PKcs promotes sepsis-induced multiple organ failure by triggering mitochondrial dysfunction. J Adv Res 41:39–48
Wang S, Zhu H, Li R, Mui D, Toan S, Chang X, Zhou H (2022) DNA-PKcs interacts with and phosphorylates Fis1 to induce mitochondrial fragmentation in tubular cells during acute kidney injury. Sci Signal 15:eabh1121
Silva JF, Olivon VC, Mestriner FLAC, Zanotto CZ, Ferreira RG, Ferreira NS, Silva CAA, Luiz JPM, Alves JV, Fazan R, Cunha FQ, Alves-Filho JC, Tostes RC (2020) Acute increase in O-GlcNAc improves survival in mice with LPS-induced systemic inflammatory response syndrome. Front Physiol. https://doi.org/10.3389/fphys.2019.01614
Chu M, Qian L, Zhu M, Yao J, Xu D, Chen M (2018) Circumferential strain rate to detect lipopolysaccharide-induced cardiac dysfunction: a speckle tracking echocardiography study. Quant Imaging Med Surg 9:151–159
Elsawy H, Almalki M, Elmenshawy O, Abdel-Moneim A (2022) In vivo evaluation of the protective effects of arjunolic acid against lipopolysaccharide-induced septic myocardial injury. PeerJ 10:e12986
Sun Y, Yao X, Zhang QJ, Zhu M, Liu ZP, Ci B, Xie Y, Carlson D, Rothermel BA, Sun Y, Levine B, Hill JA, Wolf SE, Minei JP, Zang QS (2018) Beclin-1-dependent autophagy protects the heart during sepsis. Circulation 138:2247–2262
Zhou H, Toan S, Zhu P, Wang J, Ren J, Zhang Y (2020) DNA-PKcs promotes cardiac ischemia reperfusion injury through mitigating BI-1-governed mitochondrial homeostasis. Basic Res Cardiol 115:11
Zhou H, Zhu P, Wang J, Zhu H, Ren J, Chen Y (2018) Pathogenesis of cardiac ischemia reperfusion injury is associated with CK2alpha-disturbed mitochondrial homeostasis via suppression of FUNDC1-related mitophagy. Cell Death Differ 25:1080–1093
Guo W, Jiang L, Bhasin S, Khan SM, Swerdlow RH (2009) DNA extraction procedures meaningfully influence qPCR-based mtDNA copy number determination. Mitochondrion 9:261–265
Zhou H, Wang J, Zhu P, Zhu H, Toan S, Hu S, Ren J, Chen Y (2018) NR4A1 aggravates the cardiac microvascular ischemia reperfusion injury through suppressing FUNDC1-mediated mitophagy and promoting Mff-required mitochondrial fission by CK2alpha. Basic Res Cardiol 113:23
Funding
This study was supported by grants from the NSFC (Nos. 82270279 and 82200296) and Youth Innovative Talents Training Program of Tianjin First Central Hospital Young Talents.
Author information
Authors and Affiliations
Contributions
JW and HZ conceived the original experiments. TX and YL contributed to the manuscript revision. SC and RH, and HZ, carried out all the in vivo experiments and molecular investigation in vitro. HZ and MZ wrote the whole manuscript. HZ, MZ, HZ revised the final version of manuscript. All the authors read the article and approved the submission.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared no conflict of interest.
Ethical statement
Experiments were performed under a project license (Beijing, China) granted by the committee on the Ethics of Chinese PLA General Hospital, in compliance with the Guidelines for the Management and Use of Laboratory Animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, H., Wang, J., Xin, T. et al. DUSP1 interacts with and dephosphorylates VCP to improve mitochondrial quality control against endotoxemia-induced myocardial dysfunction. Cell. Mol. Life Sci. 80, 213 (2023). https://doi.org/10.1007/s00018-023-04863-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-04863-z