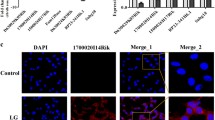
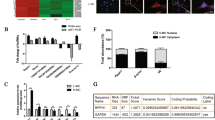
Abstract
Background
Increasing evidence indicates that N6-methyladenosine (m6A) modification of mRNAs has been shown to play a critical role in the occurrence and development of many diseases, while little is known about m6A modification in long non-coding RNAs (LncRNAs). Our study aims to investigate the potential functions of LncRNA m6A modifications in lipopolysaccharide (LPS)-induced mouse mesangial cells (MMCs), providing us with a new perspective on the molecular mechanisms of chronic glomerulonephritis (CGN) pathogenesis.
Methods
Differentially methylated LncRNAs were identified by Methylated RNA immunoprecipitation sequencing (MeRIP-seq). LncRNA–mRNA and LncRNA-associated LncRNA–miRNA–mRNA (CeRNA) networks were constructed by bioinformatics analysis. Furthermore, we utilized gene ontology (GO) and pathway enrichment analyses (KEGG) to explore target genes from co-expression networks. In addition, the total level of m6A RNA methylation and expression of methyltransferase and pro-inflammatory cytokines were detected by the colorimetric quantification method and western blot, respectively. Cell viability and cell cycle stage were detected by cell counting kit-8 (CCK-8) and flow cytometry.
Results
In total, 1141 differentially m6A-methylated LncRNAs, including 529 hypermethylated LncRNAs and 612 hypomethylated LncRNAs, were determined by MeRIP-seq. The results of GO and KEGG analysis revealed that the target mRNAs were mainly enriched in signal pathways, such as the NF-kappa B signaling pathway, MAPK signaling pathway, Toll-like receptor signaling pathway, and apoptosis signaling pathway. In addition, higher METTL3 expression was found in CGN kidney tissues using the GEO database. METTL3 knockdown in MMC cells drastically reduced the levels of m6A RNA methylation, pro-inflammatory cytokines IL6 and TNF-α, and inhibited cell proliferation and cycle progression.
Conclusions
Our findings provide a basis and novel insight for further investigations of m6A modifications in LncRNAs for the pathogenesis of CGN.







Similar content being viewed by others
Data availability
The data used and analyzed to support the findings of this study are available from the corresponding author upon request.
References
Gupta RA, Shah N, Wang KC, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464(7291):1071–6.
Ariel F, Lucero L, Christ A, et al. R-loop mediated trans action of the APOLO long noncoding RNA. Mol Cell. 2020;77(5):1055-1065.e4.
Zhang Y, Pitchiaya S, Cieślik M, et al. Analysis of the androgen receptor-regulated LncRNA landscape identifies a role for ARLNC1 in prostate cancer progression. Nat Genet. 2018;50(6):814–24.
Zhang K, Han X, Zhang Z, et al. The liver-enriched lnc-LFAR1 promotes liver fibrosis by activating TGFβ and Notch pathways. Nat Commun. 2017;8(1):144.
Li T, Hu PS, Zuo Z, et al. METTL3 facilitates tumor progression via an m6A-IGF2BP2-dependent mechanism in colorectal carcinoma. Mol Cancer. 2019;18(1):112.
Xu CL, Sang B, Liu GZ, et al. SENEBLOC, a long non-coding RNA suppresses senescence via p53-dependent and independent mechanisms. Nucleic Acids Res. 2020;48(6):3089–102.
Keihani S, Kluever V, Mandad S, et al. The long noncoding RNA neuroLNC regulates presynaptic activity by interacting with the neurodegeneration-associated protein TDP-43. Sci Adv. 2019;5(12):eaay2670.
Sun X, Haider Ali MSS, Moran M. The role of interactions of long non-coding RNAs and heterogeneous nuclear ribonucleoproteins in regulating cellular functions. Biochem J. 2017;474(17):2925–35.
Rogoyski OM, Pueyo JI, Couso JP, Newbury SF. Functions of long non-coding RNAs in human disease and their conservation in drosophila development. Biochem Soc Trans. 2017;45(4):895–904.
Desrosiers R, Friderici K, Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci U S A. 1974;71(10):3971–5.
Fustin JM, Doi M, Yamaguchi Y, et al. RNA-methylation-dependent RNA processing controls the speed of the circadian clock. Cell. 2013;155(4):793–806.
Lin S, Choe J, Du P, Triboulet R, Gregory RI. The m(6)A methyltransferase METTL3 promotes translation in human cancer cells. Mol Cell. 2016;62(3):335–45.
Wang X, Zhao BS, Roundtree IA, et al. N(6)-methyladenosine modulates messenger rna translation efficiency. Cell. 2015;161(6):1388–99.
Chen T, Hao YJ, Zhang Y, et al. m(6)A RNA methylation is regulated by microRNAs and promotes reprogramming to pluripotency. Cell Stem Cell. 2015;16(3):289–301.
Lan Q, Liu PY, Haase J, Bell JL, Hüttelmaier S, Liu T. The critical role of RNA m6A methylation in cancer. Cancer Res. 2019;79(7):1285–92.
Liu N, Zhou KI, Parisien M, Dai Q, Diatchenko L, Pan T. N6-methyladenosine alters RNA structure to regulate binding of a low-complexity protein. Nucleic Acids Res. 2017;45(10):6051–63.
Patil DP, Chen CK, Pickering BF, et al. m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature. 2016;537(7620):369–73.
Wu Y, Yang X, Chen Z, et al. m6A-induced LncRNA RP11 triggers the dissemination of colorectal cancer cells via upregulation of Zeb1. Mol Cancer. 2019;18(1):87.
Ni W, Yao S, Zhou Y, et al. Long noncoding RNA GAS5 inhibits progression of colorectal cancer by interacting with and triggering YAP phosphorylation and degradation and is negatively regulated by the m6A reader YTHDF3. Mol Cancer. 2019;18(1):143.
Sethi S, Fervenza FC. Standardized classification and reporting of glomerulonephritis. Nephrol Dial Transplant. 2019;34(2):193–9.
Chao S, Xu Q, Dong S, Guo M, Liu X, Cheng X. Polygala fallax Hemsl combined with compound Sanqi granules relieves glomerulonephritis by regulating proliferation and apoptosis of glomerular mesangial cells. J Int Med Res. 2020;48(1):300060519894124.
Shen J, Wu Q, Liang T, et al. TRIM40 inhibits IgA1-induced proliferation of glomerular mesangial cells by inactivating NLRP3 inflammasome through ubiquitination. Mol Immunol. 2021;140:225–32.
Wang Y, Mao J, Wang X, et al. Genome-wide screening of altered m6A-tagged transcript profiles in the hippocampus after traumatic brain injury in mice. Epigenomics. 2019;11(7):805–19.
Li J, Ma W, Zeng P, et al. LncTar: a tool for predicting the RNA targets of long noncoding RNAs. Brief Bioinform. 2015;16(5):806–12.
Qi X, Zhang DH, Wu N, Xiao JH, Wang X, Ma W. ceRNA in cancer: possible functions and clinical implications. J Med Genet. 2015;52(10):710–8.
Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, Benner C, Chanda SK. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10(1):1523.
Ashburner M, Ball CA, Blake JA, et al. Gene ontology: tool for the unification of biology. The Gene Ontol Consortium Nat Genet. 2000;25(1):25–9.
Dominissini D, Moshitch-Moshkovitz S, Schwartz S, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485(7397):201–6.
Qin XJ, Gao JR, Xu XJ, Jiang H, Wei LB, Jiang NN. LncRNAs expression in adriamycin-induced rats reveals the potential role of LncRNAs contributing to chronic glomerulonephritis pathogenesis. Gene. 2019;687:90–8.
Liu T, Zhuang XX, Qin XJ, Wei LB, Gao JR. Alteration of N6-methyladenosine epitranscriptome profile in lipopolysaccharide-induced mouse mesangial cells. Naunyn Schmiedebergs Arch Pharmacol. 2022;395(4):445–58.
Shushakova N, Tkachuk N, Dangers M, et al. Urokinase-induced activation of the gp130/Tyk2/Stat3 pathway mediates a pro-inflammatory effect in human mesangial cells via expression of the anaphylatoxin C5a receptor. J Cell Sci. 2005;118(Pt 12):2743–53.
Gao J, Wei L, Song J, et al. In vitro and in vivo study of the expression of the Syk/Ras/c-Fos pathway in chronic glomerulonephritis. Mol Med Rep. 2018;18(4):3683–90.
Wang Y, Li Y, Toth JI, Petroski MD, Zhang Z, Zhao JC. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat Cell Biol. 2014;16(2):191–8.
Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651.
White S, Lin L, Hu K. NF-κB and tPA signaling in kidney and other diseases. Cells. 2020;9(6):1348.
Zhang J, Yang S, Chen F, Li H, Chen B. Ginkgetin aglycone ameliorates LPS-induced acute kidney injury by activating SIRT1 via inhibiting the NF-κB signaling pathway. Cell Biosci. 2017;7:44.
Yong HY, Koh MS, Moon A. The p38 MAPK inhibitors for the treatment of inflammatory diseases and cancer. Expert Opin Investig Drugs. 2009;18(12):1893–905.
Dong Q, Jie Y, Ma J, Li C, Xin T, Yang D. Renal tubular cell death and inflammation response are regulated by the MAPK-ERK-CREB signaling pathway under hypoxia-reoxygenation injury. J Recept Signal Transduct Res. 2019;39(5–6):383–91.
Kurtzeborn K, Kwon HN, Kuure S. MAPK/ERK signaling in regulation of renal differentiation. Int J Mol Sci. 2019;20(7):1779.
Garibotto G, Carta A, Picciotto D, Viazzi F, Verzola D. Toll-like receptor-4 signaling mediates inflammation and tissue injury in diabetic nephropathy. J Nephrol. 2017;30(6):719–27.
Campbell MT, Hile KL, Zhang H, et al. Toll-like receptor 4: a novel signaling pathway during renal fibrogenesis. J Surg Res. 2011;168(1):e61–9.
Ramnath D, Powell EE, Scholz GM, Sweet MJ. The toll-like receptor 3 pathway in homeostasis, responses to injury and wound repair. Semin Cell Dev Biol. 2017;61:22–30.
Zhao SY, Liao LX, Tu PF, Li WW, Zeng KW. Icariin inhibits AGE-induced injury in PC12 cells by directly targeting apoptosis regulator bax. Oxid Med Cell Longev. 2019;2019:7940808.
Guan X, Lu J, Sun F, Li Q, Pang Y. The molecular evolution and functional divergence of lamprey programmed cell death genes. Front Immunol. 2019;10:1382.
Hughes J, Savill JS. Apoptosis in glomerulonephritis. Curr Opin Nephrol Hypertens. 2005;14(4):389–95.
Shimizu A, Masuda Y, Kitamura H, Ishizaki M, Sugisaki Y, Yamanaka N. Apoptosis in progressive crescentic glomerulonephritis. Lab Invest. 1996;74(5):941–51.
Du Y, Hou G, Zhang H, et al. SUMOylation of the m6A-RNA methyltransferase METTL3 modulates its function. Nucleic Acids Res. 2018;46(10):5195–208.
Śledź P, Jinek M. Structural insights into the molecular mechanism of the m(6)A writer complex. Elife. 2016;5:e18434.
Wang P, Doxtader KA, Nam Y. Structural Basis for Cooperative Function of Mettl3 and Mettl14 Methyltransferases. Mol Cell. 2016;63(2):306–17.
Wang X, Feng J, Xue Y, et al. Structural basis of N(6)-adenosine methylation by the METTL3-METTL14 complex. Nature. 2016;534(7608):575–8.
Ramalingam H, Kashyap S, Cobo-Stark P, et al. A methionine-Mettl3-N6-methyladenosine axis promotes polycystic kidney disease. Cell Metab. 2021;33(6):1234-1247.e7.
Wang JN, Wang F, Ke J, et al. Inhibition of METTL3 attenuates renal injury and inflammation by alleviating TAB3 m6A modifications via IGF2BP2-dependent mechanisms. Sci Transl Med. 2022;14(640):eabk2709.
Jiang L, Liu X, Hu X, et al. METTL3-mediated m6A modification of TIMP2 mRNA promotes podocyte injury in diabetic nephropathy. Mol Ther. 2022;30(4):1721–40.
Wan W, Ao X, Chen Q, et al. METTL3/IGF2BP3 axis inhibits tumor immune surveillance by upregulating N6-methyladenosine modification of PD-L1 mRNA in breast cancer. Mol Cancer. 2022;21(1):60.
Huang H, Camats-Perna J, Medeiros R, Anggono V, Widagdo J. Altered expression of the m6A methyltransferase METTL3 in Alzheimer’s disease. eNeuro. 2020. https://doi.org/10.1523/ENEURO.0125-20.2020.
Gao JR, Shi MM, Jiang H, Zhu XL, Wei LB, Qin XJ. MicroRNA-339-5p inhibits lipopolysaccharide-induced rat mesangial cells by regulating the Syk/Ras/c-Fos pathway. Naunyn Schmiedebergs Arch Pharmacol. 2022;395(9):1075–85.
Funding
This study was financially supported by the National Natural Science Foundation of China (No.81973546), the Key Scientific Research Projects of Natural Science in Colleges and Universities in Anhui Province (No.2022AH050747), and the Key Scientific Research Projects of Natural Science in Colleges and Universities in Anhui Province (No.2022AH050455).
Author information
Authors and Affiliations
Contributions
JRG conceived and designed the study. TL and XXZ wrote the paper. JRG, XJQ, and BLW reviewed and edited the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, T., Zhuang, X.X., Qin, X.J. et al. The potential role of N6-methyladenosine modification of LncRNAs in contributing to the pathogenesis of chronic glomerulonephritis. Inflamm. Res. 72, 623–638 (2023). https://doi.org/10.1007/s00011-023-01695-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-023-01695-2