Abstract
Objective
Retinal ganglion cell (RGC) apoptosis is one of the most severe complications that causes permanent visual impairment following ocular alkali burn (OAB). Currently, very few treatment options exist for this condition. This study was conducted to determine the effect of 4-phenylbutyric acid (4-PBA) on endoplasmic reticulum (ER) stress after OAB using a well-established OAB mouse model.
Methods
Ocular alkali burn was induced in C57BL/6 mouse corneas using 1 M NaOH. 4-PBA (10 mg/kg; 250 μL per injection) or saline (250 μL per injection) was injected intraperitoneally once per day for 3 days before the establishment of the OAB model. The apoptosis of retinal ganglion cells (RGCs) was assessed by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay, and the histological damage was examined by hematoxylin and eosin and immunofluorescence assay on retinal flat mounts. The key inflammatory response and the expression of ER stress-related markers in the retinal tissues were assessed by real-time PCR, western blotting and histologic analyses.
Results
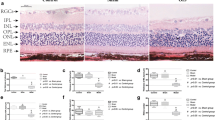
4-PBA significantly alleviated the apoptosis of RGCs and prevented the structural damage of the retina, as determined by the evaluation of RGC density and retinal thickness. Inhibition of ER stress by 4-PBA decreased the expression of vital proinflammatory cytokines, tumor necrosis factor alpha, and interleukin-1 beta; and suppressed the activation of retinal microglial cells and nuclear factor-kappa B (NF-κB). 4-PBA reduced the expression of the ER stress molecules, glucose-regulated protein 78, activated transcription factor 6, inositol-requiring enzyme-1 (IRE1), X-box-binding protein 1 splicing, and CCAAT/enhancer-binding protein homologous protein, in the retinal tissues and RGCs of OAB mice.
Conclusions
The present study demonstrated that the inhibition of ER stress by 4-PBA alleviates the inflammatory response via the IRE1/NF-κB signaling pathway and protects the retina and RGCs from injury in an OAB mouse model. Such findings further suggest that 4-PBA might have potential therapeutic implications for OAB treatment.







Similar content being viewed by others
Abbreviations
- RGCs:
-
Retinal ganglion cells
- OAB:
-
Ocular alkali burn
- 4-PBA:
-
4-Phenylbutyric acid
- ER stress:
-
Endoplasmic reticulum stress
References
Hughes WF Jr. Alkali burns of the eye; review of the literature and summary of present knowledge. Arch Ophthal. 1946;35:423–49.
Paschalis EI, et al. The role of microglia and peripheral monocytes in retinal damage after corneal chemical injury. Am J Pathol. 2018;188(7):1580–96.
Cade F, et al. Glaucoma in eyes with severe chemical burn, before and after keratoprosthesis. Cornea. 2011;30(12):1322–7.
Paschalis EI, et al. Mechanisms of retinal damage after ocular alkali burns. Am J Pathol. 2017;187(6):1327–42.
Cade F, et al. Alkali burn to the eye: protection using TNF-alpha inhibition. Cornea. 2014;33(4):382–9.
Zhou C, et al. Sustained subconjunctival delivery of infliximab protects the cornea and retina following alkali burn to the eye. Invest Ophthalmol Vis Sci. 2017;58(1):96–105.
Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–29.
Lee AS. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods. 2005;35(4):373–81.
Marciniak SJ, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18(24):3066–77.
Han J, Kaufman RJ. Physiological/pathological ramifications of transcription factors in the unfolded protein response. Genes Dev. 2017;31(14):1417–38.
Awai M, et al. NMDA-induced retinal injury is mediated by an endoplasmic reticulum stress-related protein, CHOP/GADD153. J Neurochem. 2006;96(1):43–52.
Scimone C, et al. Expression of pro-angiogenic markers is enhanced by blue light in human RPE cells. Antioxidants (Basel). 2020;9(11):1154.
Li R, et al. G-protein-coupled estrogen receptor protects retinal ganglion cells via inhibiting endoplasmic reticulum stress under hyperoxia. J Cell Physiol. 2021;236(5):3780–8.
Aoyama Y, et al. Involvement of endoplasmic reticulum stress in rotenone-induced leber hereditary optic neuropathy model and the discovery of new therapeutic agents. J Pharmacol Sci. 2021;147(2):200–7.
Hetzer SM, et al. Traumatic optic neuropathy is associated with visual impairment, neurodegeneration, and endoplasmic reticulum stress in adolescent mice. Cells. 2021;10(5):996.
Li J, et al. Endoplasmic reticulum stress is implicated in retinal inflammation and diabetic retinopathy. FEBS Lett. 2009;583(9):1521–7.
Huang H, et al. Neuroprotection by eIF2alpha-CHOP inhibition and XBP-1 activation in EAE/optic neuritiss. Cell Death Dis. 2017;8(7):e2936.
Zode GS, et al. Ocular-specific ER stress reduction rescues glaucoma in murine glucocorticoid-induced glaucoma. J Clin Invest. 2014;124(5):1956–65.
Yang L, et al. Rescue of glaucomatous neurodegeneration by differentially modulating neuronal endoplasmic reticulum stress molecules. J Neurosci. 2016;36(21):5891–903.
Doh SH, et al. Retinal ganglion cell death induced by endoplasmic reticulum stress in a chronic glaucoma model. Brain Res. 2010;1308:158–66.
Shruthi K, Reddy SS, Reddy GB. Ubiquitin-proteasome system and ER stress in the retina of diabetic rats. Arch Biochem Biophys. 2017;627:10–20.
Jeng YY, et al. Retinal ischemic injury rescued by sodium 4-phenylbutyrate in a rat model. Exp Eye Res. 2007;84(3):486–92.
Kumar V, et al. Increased ER stress after experimental ischemic optic neuropathy and improved RGC and oligodendrocyte survival after treatment with chemical chaperon. Invest Ophthalmol Vis Sci. 2019;60(6):1953–66.
Zode GS, et al. Topical ocular sodium 4-phenylbutyrate rescues glaucoma in a myocilin mouse model of primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2012;53(3):1557–65.
Zode GS, et al. Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. J Clin Invest. 2011;121(9):3542–53.
Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454(7203):455–62.
Hu P, et al. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26(8):3071–84.
Sun S, et al. Endosulfan induces endothelial inflammation and dysfunction via IRE1alpha/NF-kappaB signaling pathway. Environ Sci Pollut Res Int. 2020;27(21):26163–71.
Dohlman CH, et al. Chemical burns of the eye: the role of retinal injury and new therapeutic possibilities. Cornea. 2018;37(2):248–51.
Miyamoto F, et al. Retinal cytokine response in mouse alkali-burned eye. Ophthalmic Res. 1998;30(3):168–71.
Cheng Z, et al. Inhibition of Notch1 signaling alleviates endotoxin-induced inflammation through modulating retinal microglia polarization. Front Immunol. 2019;10:389.
Rojas B, et al. Microglia in mouse retina contralateral to experimental glaucoma exhibit multiple signs of activation in all retinal layers. J Neuroinflammation. 2014;11:133.
Walter L, Neumann H. Role of microglia in neuronal degeneration and regeneration. Semin Immunopathol. 2009;31(4):513–25.
Kyuhou S, Kato N, Gemba H. Emergence of endoplasmic reticulum stress and activated microglia in Purkinje cell degeneration mice. Neurosci Lett. 2006;396(2):91–6.
Cui R, et al. Methane-rich saline alleviates CA/CPR brain injury by inhibiting oxidative stress, microglial activation-induced inflammatory responses, and ER stress-mediated apoptosis. Oxid Med Cell Longev. 2020;2020:8829328.
Harvey LD, et al. Administration of DHA reduces endoplasmic reticulum stress-associated inflammation and alters microglial or macrophage activation in traumatic brain injury. ASN Neuro. 2015;7(6):1759091415618969. https://doi.org/10.1177/1759091415618969.
Hu Y, et al. Differential effects of unfolded protein response pathways on axon injury-induced death of retinal ganglion cells. Neuron. 2012;73(3):445–52.
Kaneko M, Niinuma Y, Nomura Y. Activation signal of nuclear factor-kappa B in response to endoplasmic reticulum stress is transduced via IRE1 and tumor necrosis factor receptor-associated factor 2. Biol Pharm Bull. 2003;26(7):931–5.
Sprenkle NT, et al. Endoplasmic reticulum stress and inflammation in the central nervous system. Mol Neurodegener. 2017;12(1):42.
Lindquist SL, Kelly JW. Chemical and biological approaches for adapting proteostasis to ameliorate protein misfolding and aggregation diseases: progress and prognosis. Cold Spring Harb Perspect Biol. 2011;3(12):a004507.
Collins AF, et al. Oral sodium phenylbutyrate therapy in homozygous beta thalassemia: a clinical trial. Blood. 1995;85(1):43–9.
Maestri NE, et al. Long-term treatment of girls with ornithine transcarbamylase deficiency. N Engl J Med. 1996;335(12):855–9.
Dover GJ, Brusilow S, Charache S. Induction of fetal hemoglobin production in subjects with sickle cell anemia by oral sodium phenylbutyrate. Blood. 1994;84(1):339–43.
Zode GS, et al. Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. J Clin Invest. 2015;125(8):3303.
Yoshikawa A, et al. Deletion of Atf6alpha impairs astroglial activation and enhances neuronal death following brain ischemia in mice. J Neurochem. 2015;132(3):342–53.
Dasgupta S, et al. Sodium phenylacetate inhibits adoptive transfer of experimental allergic encephalomyelitis in SJL/J mice at multiple steps. J Immunol. 2003;170(7):3874–82.
Wang Z, et al. Endoplasmic reticulum stress-induced neuronal inflammatory response and apoptosis likely plays a key role in the development of diabetic encephalopathy. Oncotarget. 2016;7(48):78455–72.
Guzman Mendoza NA, et al. Neuroprotective effect of 4-phenylbutyric acid against photo-stress in the retina. Antioxidants (Basel). 2021;10(7):1147.
Li H, et al. 4-Phenylbutyric acid protects against ethanol-induced damage in the developing mouse brain. Alcohol Clin Exp Res. 2019;43(1):69–78.
Salminen A, et al. ER stress in Alzheimer’s disease: a novel neuronal trigger for inflammation and Alzheimer’s pathology. J Neuroinflammation. 2009;6:41.
Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7(12):1013–30.
Hasnain SZ, et al. The interplay between endoplasmic reticulum stress and inflammation. Immunol Cell Biol. 2012;90(3):260–70.
Acknowledgements
This work is supported by National Natural Science Foundation of China (81974135, 81900851) and the Fundamental Research Funds of the State Key Laboratory of Ophthalmology (30306020240020130, 3030902113030).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All experiments followed the US National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals developed by the US National Academy of Sciences and were approved by the Administration Committee of Experimental Animals, Guangdong Province, China.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11_2022_1565_MOESM2_ESM.jpg
Supplementary file2 Effect of three different concentrations of 4-PBA on RGC apoptosis in the retinal tissues of OAB mice. A Representative retinal cryosection images stained with TUNEL (red fluorescence) and DAPI (blue) in the saline treatment group, 10 mg/kg/d 4-PBA treatment group, 40 mg/kg/d 4-PBA treatment group, and 80 mg/kg/d 4-PBA treatment group on day one after OAB. B Quantitative analysis of the TUNEL+ cells in the different groups in the central retina, mid-peripheral retina, and peripheral retina. Data are expressed as mean ± SEM (*P<0.05, **P<0.01, ***P<0.001). Scale bar = 20 μm. OAB, ocular alkali burn; 4-PBA, 4-phenylbutyric acid; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer (JPG 11530 KB)
11_2022_1565_MOESM3_ESM.jpg
Supplementary file3 The mRNA transcription and protein levels of the ER stress markers and inflammatory factors within 24 hours after OAB. The expression levels of the ER stress-related molecules, GRP78 (A) and CHOP (B), and inflammatory cytokines, TNF-α (C) and IL-1β (D), in the OAB retinal tissues at 6, 12, and 24 hours after OAB were analyzed by RT-PCR. Representative gel images and quantification graphs for GRP78 (E) and CHOP (F). The optical density of each band was normalized by the relative β-tubulin level. Data are expressed as mean ± SEM (*P < 0.05, **P < 0.01). Ctrl, control; OAB, ocular alkali burn (JPG 3199 KB)
Rights and permissions
About this article
Cite this article
Huang, Y., Yuan, M., Duan, F. et al. Inhibition of endoplasmic reticulum stress by 4-phenylbutyrate alleviates retinal inflammation and the apoptosis of retinal ganglion cells after ocular alkali burn in mice. Inflamm. Res. 71, 577–590 (2022). https://doi.org/10.1007/s00011-022-01565-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-022-01565-3