Abstract
Background
It has previously been observed that the insulin-producing cells of human pancreatic islets are more resistant to alloxan-, streptozotocin-, nitroprusside-, or cytokine-induced injury than those of mouse and rat islets.
Materials and Methods
Human pancreatic islets were obtained from heart-beating organ donors. The expression of the stress proteins heat shock protein 70 (hsp70) and heme oxygenase and the anti-apoptosis gene bcl-2 was determined in isolated rat, mouse, and human islets, either cultured in vitro or transplanted under the kidney capsule of nude mice, using immunoblot analysis. Rat and human islet sensitivity to hydrogen peroxide was assessed by glucose oxidation measurements. Isolated islets were also analyzed for their catalase and superoxide dismutase activities, and the islet cell levels of reduced glutathione were determined in response to hydrogen peroxide and nitroprusside. Programmed cell death in human and rat islets in response to streptozotocin was evaluated using TUNEL staining.
Results
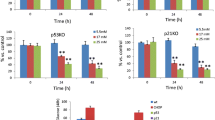
Cultured human islets expressed higher contents of hsp70 than mouse and rat islets at basal conditions. Also after 4 weeks under the kidney capsule of normoglycemic mice, the hsp70 levels were higher in human islets than in rat islets. The expression of another stress protein, heme oxygenase (HO), was strongly increased in cultured rat islets, but was not affected in human islets. Expression of the bcl-2 gene could not be detected in human islets. In spite of this, 0.5 mM streptozotocin induced apotosis in rat but not in human islet cells. Hydrogen peroxide (0.1 and 0.4 mM) decreased glucose oxidation rates in rat but not in human islets. The levels of reduced glutathione were moderately decreased in human and rat islet cells and sharply decreased in mouse islet cells in response to hydrogen peroxide. Moreover, the activities of catalase and superoxide dismutase (SOD) were markedly lower in mouse islets than in human islets. The activity of catalase was lower in rat islets than in human islets.
Conclusions
Human islets differ clearly from mouse and rat islets in their increased expression of hsp70, catalase, and SOD, which may explain the increased resistance of human islets to β cell toxins.









Similar content being viewed by others
References
Eizirik DL, Sandler S, Palmer JP. (1993) Repair of pancreatic β-cells, a relevant phenomenon in early IDDM? Diabetes 42: 1383–1391.
Eizirik DL, Pipeleers DG, Zhidong L, Welsh N, Hellerström C, Andersson A. (1994) Major species differences between humans and rodents in the susceptibility to pancreatic β-cell injury. Proc. Natl. Acad. Sci. U.S.A. 91: 9253–9256.
Eisenbarth GS. (1986) Type I diabetes. A chronic autoimmune disease. N. Engl. J. Med. 314: 1360–1368.
Skyler JS, Marks JB. (1993) Immune intervention in type I diabetes mellitus. Diabetes Rev. 1: 15–42.
Eizirik DL, Sandler S, Welsh N, et al. (1994) Cytokines suppress human islet function irrespective of their effects on nitric oxide generation. J. Clin. Invest. 93: 1968–1974.
Craig EA. (1985) The heat shock response. CRC Crit. Rev. Biochem. 18: 239–280.
Margulis BA, Sandler S, Eizirik DL, Welsh N, Welsh M. (1991) Liposomal delivery of purified heat shock protein hsp70 into rat pancreatic islets as protection against interleukin-1β induced impaired β-cell function Diabetes 40: 1418–1422.
Helqvist S, Polla BS, Johannesen J, Nerup J. (1991) Heat shock protein induction in rat pancreatic islets by recombinant human interleukin 1β Diabetologia 34: 150–156.
Welsh N, Welsh M, Lindquist S, Eizirik DL, Bendtzen K, Sandler S. (1991) Interleukin-1β increases the biosynthesis of the heat shock protein hsp70 and selectively decreases the biosynthesis of five proteins in rat pancreatic islets. Autoimmunity 9: 33–40.
Welsh N, Sandler S. (1994) Protective action by hemin against interleukin-1β induced inhibition of rat pancreatic islet function. Mol. Cell. Endocrinol. 103: 109–114.
Deneke SM, Fanburg BL. (1989) Regulation of cellular glutathione. Am. J. Phys. 257: L163–L173.
Grankvist K, Marklund S, Täljedal I-B. (1981) CuZn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochem. J. 199: 393–398.
Malaisse WJ, Malaisse-Lagae F, Sener A, Pipeleers DG. (1982) Determinants of the selective toxicity of alloxan to the pancreatic B-cell. Proc. Natl. Acad. Sci. U.S.A. 79: 827–930.
Eizirik DL, Korbutt GS, Hellerström C. (1992) Prolonged exposure of human pancreatic islets to high glucose concentrations in vitro impairs the β-cell function. J. Clin. Invest. 90: 1263–1268.
Pipeleers DG, In’t Veld P, Van de Winkel M, Maes E, Schuit F, Gepts W. (1985) A new in vitro model for the study of pancreatic A and B cells. Endocrinology 117: 806–816.
Andersson A. (1978) Isolated mouse pancreatic islets in culture: effects of serum and different culture media on the insulin production of the islets. Diabetologia 14: 397–404.
Welsh N, Hellerström C. (1990) In vitro restoration of insulin production in islets from adult rats treated neonatally with streptozotocin. Endocrinology 126: 1842–1848.
Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254.
Margulis BA, Nacharov PV, Tsvetkova OI, Welsh M, Kinev AV. (1991) The characterization and use of different antibodies against the hsp70 major heat shock protein family for the development of an immunoassay. Electrophoresis 12: 670–673.
Welch WJ, Feramisco JR. (1985) Rapid purification of mammalian 70000-dalton stress proteins: Affinity of the proteins for nucleotides. Mol. Cell. Biol. 5: 1229–1237.
Pezzella F, Tse AG, Cordell JL, Polford KA, Gatter KC, Mason DY. (1990) Expression of the bcl-2 oncogene protein is not specific for the 14:18 chromosomal translocation. Am. J. Pathol. 137: 225–232.
Mellgren A, Schnell Landström AH, Petersson B, Andersson A. (1986) The renal subcapsular site offers better growth conditions for transplanted mouse pancreatic islet cells than the liver or spleen. Diabetologia 29: 670–672.
Shrieve DC, Bump EA, Rice GC. (1988) Heterogeneity of cellular glutathione among cells derived from a murine fibrosarcoma or a human renal cell carcinoma detected by flow cytometric analysis. J. Biol. Chem. 263: 14107–14114.
Johansson LH, Borg LAH. (1988) A spectrophotometric method for determination of catalase activity in small tissue samples. Anal. Biochem. 174: 331–336.
Eriksson UJ, Borg LAH. (1991) Protection by free oxygen radical scavenging enzymes against glucose-induced embryonic malformations in vitro. Diabetologia 34: 325–331.
Pettersson M, Jernberg Wiklund H, Larsson L-G, Nilsson K. (1992) Expression of the bcl-2 gene in human multiple myeloma cells lines and normal plasma cells. Blood 79: 495–502.
Moreira JE, Hand AR, Borg LAH, et al. (1991) Decrease of insulin-containing secretory granules and mitochondrial gene expression in mouse pancreatic islets maintained in culture following streptozotocin exposure. Virchows Arch. 60: 337–344.
Greene JM, Larin Z, Taylor ICA, Prentice H, Gwinn KA, Kingston RE. (1987) Multiple basal elements of a human hsp70 promoter function differently in human and rodent cell lines. Mol. Cell. Biol. 7: 3646–3655.
Bensaude O, Morange M. (1983) Spontaneous high expression of heat shock proteins in mouse embryonal carcinoma cells and ectoderm from day 8 mouse embryo. EMBO J. 2: 173–177.
Stoker R. (1990) Induction of heme oxygenase as a defence against oxidative stress. Free Radic. Res. Commun. 9: 101–112.
Borg LAH, Cagliero E, Sandler S, Welsh N, Eizirik DL. (1992) Interleukin-1β increases the activity of superoxide dismutase in rat pancreatic islets. Endocrinology 130: 2851–2857.
Pociot F, Rønningen KS, Nerup J. (1993) Polymorphic analysis of the human MHC-linked heat shock protein 70 (HSP70-2) and HSP70-Hom genes in insulin-dependent diabetes mellitus (IDDM). Scand. J. Immunol. 38: 491–495.
Llesuy SF, Tomaro ML. (1994) Heme oxygenase and oxidative stress. Evidence of involvement of bilirubin as physiological protector against oxidative damage. Biochem. Biophys. Acta 1223: 9–14.
Vile GF, Basu-Modak S, Waltner C, Tyrrell RM. (1994) Heme oxygenase 1 mediates an adaptive response to oxidative stress in human skin fibroblasts. Proc. Natl. Acad. Sci. U.S.A. 91: 2607–2610.
Raju VS, Maines MD. (1994) Coordinated expression and mechanism of induction of HSP32 (heme oxygenase-1) mRNA by hyperthermia in rat organs. Biochim. Biophys. Acta 1217: 273–280.
Cornelius JG, Luttge BG, Peck AB. (1993) Antioxidant enzyme activities in IDD-prone and IDD-resistant mice: A comparative study. Free Radic. Biol. Med. 14: 409–420.
Horio F, Fukuda M, Katoh H, et al. (1994) Reactive oxygen intermediates in autoimmune islet cell destruction of the NOD mouse induced by peritoneal exudate cells (rich in macrophages) but not T cells. Diabetologia 37: 22–31.
Andersen HU, Jørgensen KH, Egeberg J, Mandrup-Poulsen T, Nerup J. (1994) Nicotinamide prevents interleukin-1 effects on accumulated insulin release and nitric oxide production in rat islets of Langerhans. Diabetes 43: 770–777.
Wilson GL, Patton NJ, McCord JM, Mullins DW, Mossman BT. (1984) Mechanisms of streptozotocin- and alloxan-induced damage in rat β-cells. Diabetologia 27: 587–591.
Eizirik DL, Sandler S, Welsh N, Bendtzen K, Hellerström C. (1994) Nicotinamide decreases nitric oxide production and partially protects human pancreatic islets against the suppressive effects of combinations of cytokines. Autoimmunity 19: 193–198.
Juntti-Berggren L, Larsson O, Rorsman P, et al. (1993) Increased activity of L-type Ca2+ channels exposed to serum from patients with type I diabetes. Science 261: 86–90.
Lorenzo A, Razzabone B, Weir GC, Yankner BA. (1994) Pancreatic islet cell toxicity of amylin associated with type 2-diabetes mellitus. Nature 368: 756–760.
Morgan NG, Cable HC, Newcombe NR, Williams GT. (1994) Treatment of cultured pancreatic B-cells with streptozotocin induces cell death by apoptosis. Biosci. Rep. 14: 243–250.
Vaux DL, Cory S, Adams JM. (1988) Bcl-2 gene promotes haematopoetic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 335: 440–442.
Strasser A, Harris AW, Cory S. (1991) Bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell 67: 889–899.
Tsujimoto Y. (1989) Stress-resistance conferred by high level of bcl-2 alpha protein in human lymphoblastoid cell. Oncogene 4: 1331–1336.
Acknowledgments
The excellent technical assistance of C. Göktürk, I.-B. Hallgren, E. Törnelius, E. Forsbeck, A. Nordin, and M. Engkvist is acknowledged. This study made use of human islets prepared by the Central Unit of the β Cell Transplant, with financial support of a concerted action in Medical and Health Research of the European Community. The study was also supported by the Swedish Medical Research Council (Grant 12X-9237, associated with the β Cell Transplant, European Concerted Action for the Treatment of Diabetes; and Grants 12X-109, 12X-9886, 12X-6538, 12P-10151, and 19P-8982), the Juvenile Diabetes Foundation International, the Swedish Diabetes Association, the Novo-Nordisk Insulin Foundation Committee, the Swedish CFN (Forskningsanlag för alternativa metoder), the Family Ernfors Fund, BIOMED concerted action, the Vlaamse Gemeenschap (Grant 9297-1807), and the Belgian Fonds voor Geneeskundig Wetenschappelijk Onderzoek (Grant 3.0057.94). Maria Alice R. de Mello is on leave from the Departamento de Educacao Fisica, UNESP, Rio Claro, SP, Brazil. She is the recipient of a postdoctoral fellowship from the Brazilian Research Agency CNPQ.
Author information
Authors and Affiliations
Additional information
Contributed by D. F. Steiner on July 5, 1995.
Rights and permissions
About this article
Cite this article
Welsh, N., Margulis, B., Borg, L.A.H. et al. Differences in the Expression of Heat-Shock Proteins and Antioxidant Enzymes between Human and Rodent Pancreatic Islets: Implications for the Pathogenesis of Insulin-Dependent Diabetes Mellitus. Mol Med 1, 806–820 (1995). https://doi.org/10.1007/BF03401895
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03401895